110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

替加环素与亚胺培南/西司他丁治疗中国住院患者并发腹腔感染的疗效和安全性的多中心、双盲、随机对照研究
Authors Chen Y, Zhu D, Zhang Y, Zhao Y, Chen G, Li P, Xu L, Yan P, Hickman MA, Xu X, Tawadrous M, Wible M
Received 20 April 2018
Accepted for publication 22 September 2018
Published 30 November 2018 Volume 2018:14 Pages 2327—2339
DOI https://doi.org/10.2147/TCRM.S171821
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 3
Editor who approved publication: Professor Deyun Wang
Purpose: To assess the efficacy and safety of
tigecycline in treating complicated intra-abdominal infections (cIAIs) in
hospitalized patients in China.
Patients and methods: A Phase IV, multicenter, randomized, double-blinded,
active-controlled, non-inferiority study was conducted. Hospitalized cIAI
patients ≥18 years of age were randomized (1:1) to receive intravenous
tigecycline (initial dose 100 mg, then 50 mg q12h) or imipenem/cilastatin (500
mg/500 mg or adjusted for renal dysfunction, q6h) for 5–14 days. The primary
end point was clinical response for clinically evaluable (CE) subjects at
test-of-cure (TOC) assessment.
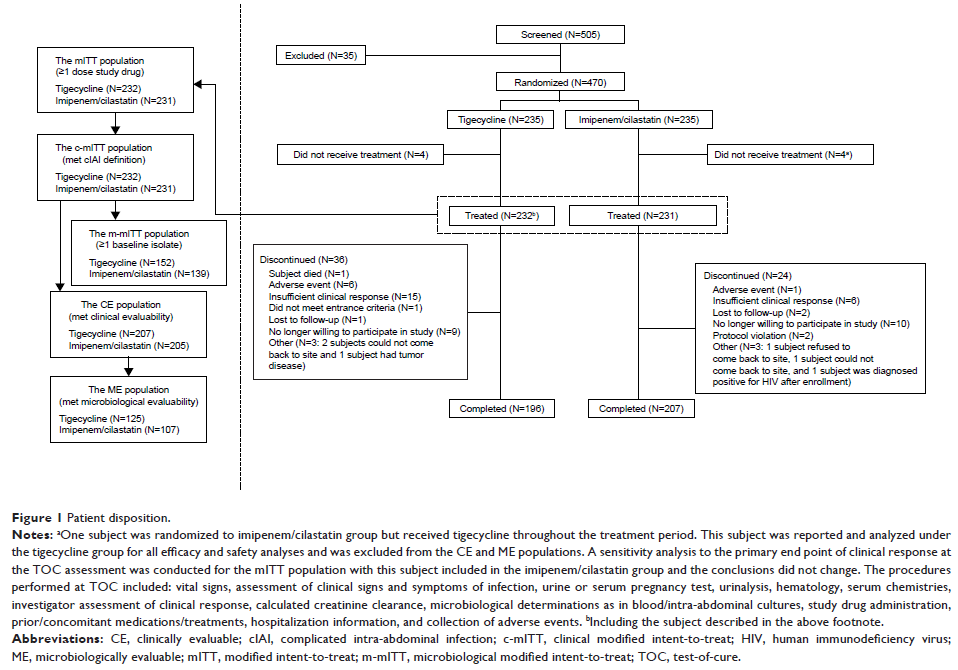
Results: Four
hundred and seventy subjects were randomized; 232 in the tigecycline and 231 in
the imipenem/cilastatin group were treated. Tigecycline was non-inferior to
imipenem/cilastatin with respect to clinical response at TOC for all CE
subjects, ie, the lower bound of the two-sided 95% CI (−12.0%, −1.4%) for the
treatment difference in cure rate, tigecycline (89.9%) minus
imipenem/cilastatin (96.6%), was >−15%. As non-inferiority was concluded in
the CE population, superiority of tigecycline over imipenem/cilastatin and
superiority of imipenem/cilastatin over tigecycline were tested on the CE and
the modified intent-to-treat (mITT) populations according to pre-specified
statistical criteria, and neither could be demonstrated (the cure rate was
82.8% vs 88.7%, difference -6.0% [−12.8%, 0.8%], for the mITT population).
The subject-level microbiological response rate at TOC for the
microbiologically evaluable population was 88.0% (110/125) vs 95.3%
(102/107, difference -7.3% [−15.2%, 0.5%]). Nausea, drug ineffectiveness, postoperative
wound infection, vomiting, and pyrexia were the most common adverse events in
tigecycline-treated subjects; pyrexia, nausea, vomiting, and increased alanine
aminotransferase and aspartate aminotransferase levels were most common in
imipenem/cilastatin-treated subjects; none were unanticipated.
Conclusion: Tigecycline
was non-inferior to imipenem/cilastatin in treating hospitalized adult patients
with cIAI. Superiority of tigecycline over imipenem/cilastatin or
imipenem/cilastatin over tigecycline could not be demonstrated. Safety was
consistent with the known profile for tigecycline.
ClinicalTrials.gov identifier: NCT01721408.
Keywords: tigecycline,
imipenem/cilastatin, complicated intra-abdominal infections, non-inferiority