110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

生物粘附性屏障形成口服液对化疗和/或放疗引起的口腔粘膜炎的癌症患者的局部镇痛作用:一个多中心、随机、一次性、开放、阳性对照标签研究
Authors Cheng Y, Qin SK, Chen YP, Dong LH, Sun XD, Yu SY, Wu SK
Received 30 August 2018
Accepted for publication 31 October 2018
Published 30 November 2018 Volume 2018:11 Pages 8555—8564
DOI https://doi.org/10.2147/OTT.S185915
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Narasimha Reddy Parine
Peer reviewer comments 3
Editor who approved publication: Dr Takuya Aoki
Objective: CAM2028
(Episil®; Camurus AB, Lund, Sweden) is a liquid for use
in the oral cavity to treat various pains associated with mouth injuries. Upon
contact with the swollen oral mucosa, the oral liquid forms a thin protective
film that acts as a mechanical barrier to relieve pain. This study was the
first in China to evaluate the local analgesic effect of oral liquid in cancer
patients who developed oral mucositis following chemotherapy and/or
radiotherapy.
Methods: A total
of 60 patients were randomized in a 1:1 ratio to the CAM2028 group (the pump
device was firmly pressed three times and the fluid was distributed to the
painful area of the oral cavity) or KS (a mucoadhesive oral wound rinse,
Kangsu™; Luye Pharmaceutical Co. Ltd, Nanjing, China) group (5 mL of the
oral rinse was poured into and kept in the oral cavity for at least 1 minute).
The primary endpoint was the area under the oral mucosal pain score–time curve
(AUC) within 6 hours of treatment in the trial and control groups. Medical
device adverse events were assessed according to the National Cancer
Institute’s Common Terminology Criteria for Adverse Events, version 4.0.
Statistical analyses were performed using the chi-squared test (Fisher’s exact
test), independent-samples t-test, and analysis of covariance.
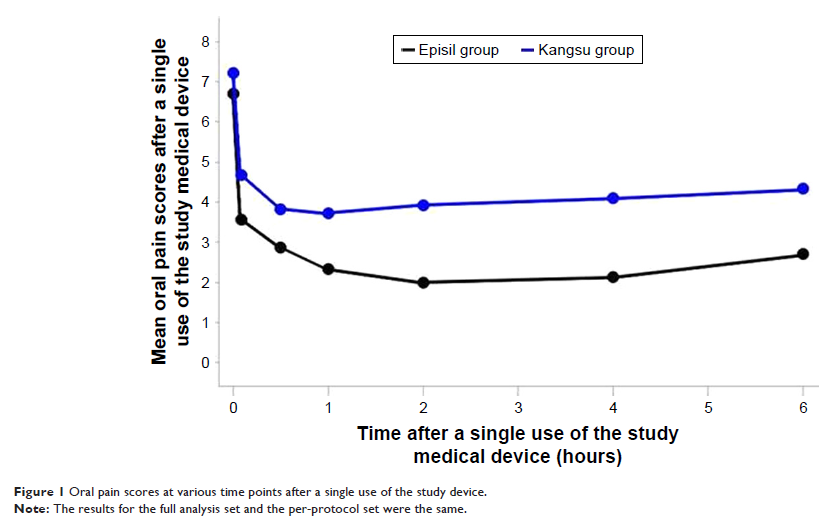
Results: Sixty patients
were included in the per-protocol set population analysis. The average
(mean ± SD) 6-hour AUC of the CAM2028 group and the KS group was
14.20±10.29 and 24.46±14.15, respectively. The difference between the groups
was statistically significant (P =0.0022). The incidence of adverse events in the
trial group and the control group was 16.67% and 30.0%, respectively, and there
was no statistical difference.
Conclusions: CAM2028
displayed an efficacious local analgesic effect in cancer patients who
developed oral mucositis following chemotherapy and/or radiotherapy. The
results demonstrated its potential value in clinical applications.
Keywords: oral
mucositis, radiotherapy, chemotherapy, analgesic effect, episil