110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

以贝伐单抗为主体的联合疗法用于治疗转移性结直肠癌的疗效和安全性
Authors Xu R, Xu C, Liu C, Cui C, Zhu J
Received 20 April 2018
Accepted for publication 3 September 2018
Published 4 December 2018 Volume 2018:11 Pages 8605—8621
DOI https://doi.org/10.2147/OTT.S171724
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Yao Dai
Aim: The use
of bevacizumab in combination therapy is an emerging trend in metastatic
colorectal cancer treatment. However, the clinical value of different
combination types remains under debate. Thus, a meta-analysis of randomized
controlled trials (RCTs) comparing bevacizumab-based combination therapy with
monotherapy (therapy that uses one type of treatment, such as chemotherapy or
surgery alone, to treat metastatic colorectal cancer) was performed, aiming to
evaluate the safety and efficacy of bevacizumab-based combination therapy and
to find a more beneficial combination.
Methods: We
searched for clinical studies that evaluated bevacizumab-based combination
therapy in metastatic colorectal cancer. We extracted data from these studies
to evaluate the relative risk (RR) of overall response rate (ORR) and grade 3/4
treatment-related adverse events (AEs), HRs of overall survival (OS), and
progression-free survival (PFS).
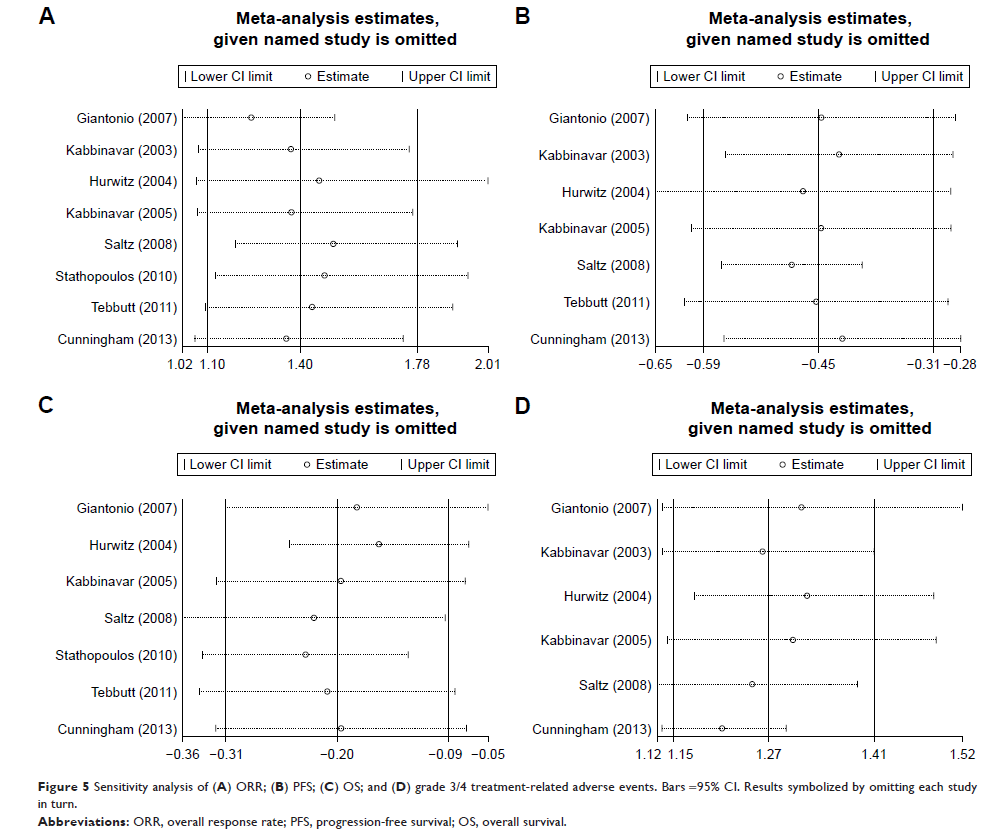
Results: Eight
RCTs were identified (n=3,424). Treatments included combinations of bevacizumab
and oxaliplatin, fluorouracil, and leucovorin (FOLFOX4), combinations of
bevacizumab and capecitabine and oxaliplatin, combinations of bevacizumab and
fluorouracil/leucovorin, combinations of bevacizumab and irinotecan,
fluorouracil, and leucovorin (IFL), and combinations of bevacizumab and
capecitabine. Bevacizumab-based combination therapy showed higher ORR (RR:
1.40; 95% CI: 1.10–1.78; P =0.005), PFS (HR: 0.64; 95% CI: 0.55–0.73; P =0.000), and OS
(HR: 0.82; 95% CI: 0.73–0.92; P =0.001) values than monotherapy. However, higher
grade 3/4 treatment-related AEs (RR: 1.27; 95% CI: 1.15–1.41; P =0.000) were
observed in combination therapy than in monotherapy.
Conclusion: This
meta-analysis showed that the addition of IFL to bevacizumab better benefits
PFS and safety. Adding FOLFOX4 was associated with better ORR and OS. The
efficacy and safety of an IFL–bevacizumab–FOLFOX4 combination should be given
greater weight in future clinical trials, guidelines, and clinical practice.
Keywords: combination
therapy, bevacizumab, metastatic colorectal cancer, meta-analysis