110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

FAM3C 表达升高可促进胃癌中的细胞上皮 - 间质转化和细胞迁移
Authors Shi M, Duan G, Nie S, Shen S, Zou X
Received 28 June 2018
Accepted for publication 26 September 2018
Published 5 December 2018 Volume 2018:11 Pages 8491—8505
DOI https://doi.org/10.2147/OTT.S178455
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Justinn Cochran
Peer reviewer comments 2
Editor who approved publication: Dr William Cho
Background: Tumor
metastasis is an important factor in treatment failure for advanced gastric
cancer. Family with sequence similarity 3 member C (FAM3C) is known to play a
critical role in inducing epithelial–mesenchymal transition in several cancer
types, while its role in gastric cancer is unidentified. The aim of this study
was to investigate the role of FAM3C in gastric cancer and provide new
information on the receptor tyrosine-kinase pathway and cytokine-based
therapies.
Methods: FAM3C
expression was tested in human gastric cancer tissue and adjacent normal
mucosa, and the prognostic effect of FAM3C was analyzed in data from the Cancer
Genome Atlas (TCGA). The role of FAM3C in gastric cancer proliferation and
metastasis was investigated in vitro and in vivo. Western blot analysis and
immunofluorescence were used to detect the underlying mechanisms.
Results: FAM3C
expression was increased in gastric cancer tissue and showed cytoplasmic
distribution. Gastric cancer patients with FAM3C overexpression had
significantly worse prognoses based on TCGA data. In the gastric cancer cell
lines MKN45 and AGS, knockdown of FAM3C dramatically attenuated cell migration,
but had almost no influence on proliferation, while exogenous FAM3C promoted
cell migration in a cell line with low FAM3C expression. Kyoto Encyclopedia of
Genes and Genomes (KEGG) pathway enrichment of TCGA data showed that FAM3C was
mainly associated with genes involved in focal adhesion, extracellular
matrix–receptor interactions and the PI3K–Akt signaling pathway. Knockdown
of FAM3C in
gastric cancer cell lines significantly suppressed epithelial–mesenchymal
transition, as demonstrated by increased expression of E-cadherin and decreased
expression of Snail and Slug. Furthermore, knockdown of FAM3C strongly
suppressed activation of the PI3K–Akt signaling pathway. Finally, we confirmed
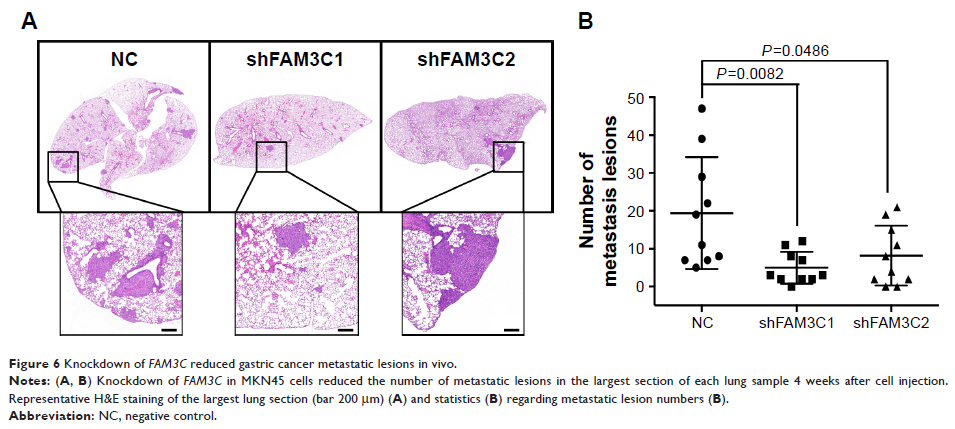
that FAM3C knockdown
significantly decreased metastatic lesions in vivo.
Conclusion: Our study
demonstrated that FAM3C can promote gastric cancer metastasis both in vitro and
in vivo. FAM3C should be taken into consideration for gastric cancer treatments
involving inhibition of the ligands and downstream pathways of receptor
tyrosine kinases.
Keywords: FAM3C,
EMT, gastric cancer, metastasis