110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

阿瓦替尼治疗尤文氏肉瘤的疗效和安全性:一家机构的回顾性分析
Authors Wang Y, Min L, Zhou Y, Luo Y, Duan H, Tu C
Received 22 July 2018
Accepted for publication 26 September 2018
Published 11 December 2018 Volume 2018:10 Pages 6835—6842
DOI https://doi.org/10.2147/CMAR.S181087
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Background: Ewing’s
sarcoma (ES) is a highly aggressive and metastatic neoplasm occurring mainly in
children and young adults. The standard treatment of localized ES requires a
combination of surgery, chemotherapy, and radiotherapy. Although the 5-year
survival rate for local ES has improved, the survival rate and prognosis are
still very poor for metastatic or recurrent ES patients. The aim of this study
was to investigate the efficacy and safety of apatinib, a specific vascular
endothelial growth factor receptor 2 inhibitor, in ES patients.
Methods: This
retrospective analysis involved eleven patients with ES not amenable to
curative treatment. All patients suffered poor responses to two cycles of
chemotherapy (vincristine, doxorubicin, and cyclophosphamide). Apatinib 500 mg
(or 250 mg) was given daily. Tumor responses were assessed according to the
Response Evaluation Criteria in Solid Tumors 1.1. Survival analysis was
performed by the Kaplan–Meier test. The safety profile was also recorded.
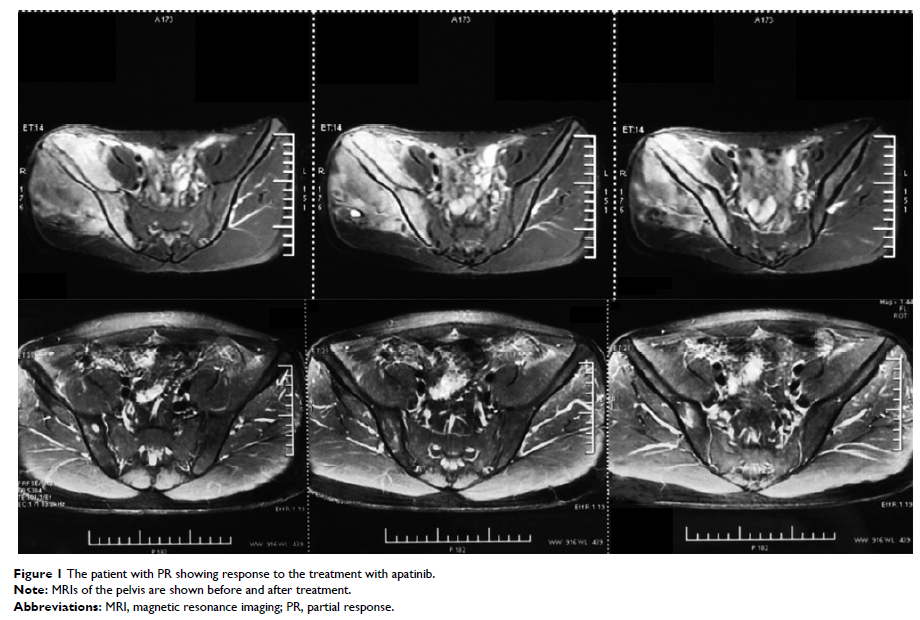
Results: The mean
age of the patients was 18 (range, 10–31) years. The 12-month overall survival
and progression-free survival rates were 90% and 72%, respectively. Four
patients achieved partial response, and four patients achieved stable disease,
with objective response rate of 40%. The median follow-up in our study was 16
months (range, 3–26 months). The most common adverse events included hand–foot
skin reaction (n=5; 45%), oral ulcers (n=4; 36%), and gastrointestinal
discomfort (n=4; 36%).
Conclusion: Apatinib
may provide as second- or first-line treatment options for ES patients,
particularly in chemoresistant cases. Further studies with more cases and
longer follow-up will be necessary to determine the clinical efficacy and
safety of apatinib in ES patients.
Keywords: Ewing’s
sarcoma, apatinib, vascular endothelial growth factor, efficacy, safety