110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

奥希替尼治疗非小细胞肺癌的疗效和安全性:对试验证据的综合分析
Authors Chen P, Chen F, Lei J, Zhou B
Received 31 July 2018
Accepted for publication 25 November 2018
Published 12 December 2018 Volume 2018:11 Pages 9033—9047
DOI https://doi.org/10.2147/OTT.S182077
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Jianmin Xu
Background: Osimertinib
is an EGFR-TKI that is selective for both EGFR-TKI-sensitizing and T790M
resistance mutations in patients with non-small-cell lung cancer (NSCLC). The
purpose of this study was conducting a meta-analysis to evaluate the clinical
efficacy and safety of osimertinib in the treatment for NSCLC.
Methods: Using
“osimertinib” as a keyword combined with “non-small-cell lung cancer” and
“randomized controlled trial” as medical subject headings, the following
electronic databases were searched: PubMed, EMBASE, Cochrane Library, and China
National Knowledge Infrastructure. After data extraction and quality assessment
of the included randomized controlled trials, the RevMan 5.3 software and R
meta package were applied for meta-analysis of objective response rate (ORR),
disease control rate (DCR), progression-free survival (PFS), overall survival
(OS), and safety.
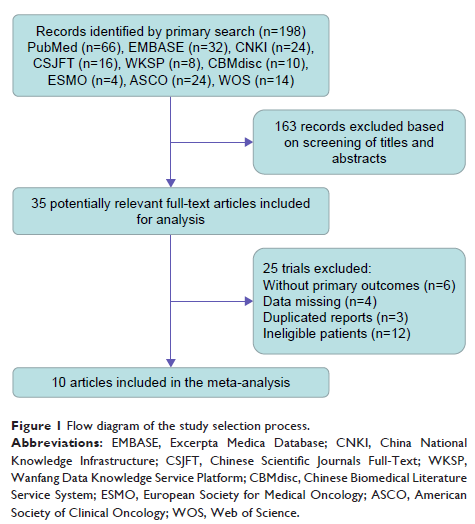
Results: Ten
studies met our criteria and were included in the meta-analysis, with a total
of 3,260 participants. The meta-analysis showed that osimertinib therapy was
superior to the control therapy alone in ORR (combined RR=1.53, 95% CI:
0.87–2.71, P =0.14),
DCR (combined RR=1.07, 95% CI: 0.79–1.44, P =0.66), PFS
(combined RR=0.32, 95% CI: 0.24–0.44, P <0.00001), and OS (combined RR=0.57, 95% CI:
0.47–0.70, P <0.00001).
In addition, osimertinib led to some toxicities, and the overall prevalence of
all-grade diarrhea was 40% (95% CI: 33–47), paronychia 26% (95% CI: 20–33),
rash 40% (95% CI: 34–47), dry skin 28% (95% CI: 23–33), and stomatitis 15% (95%
CI: 9–23).
Conclusion: Our study
showed that osimertinib demonstrated a significant improvement in the ORR, DCR,
PFS, and OS with tolerable adverse effects for NSCLC patients. However, because
of some clear limitations (heterogeneity and publication bias), these results
should be interpreted with caution.
Keywords: osimertinib,
NSCLC, efficacy, safety, survival, meta-analysis