110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

左乙拉西坦治疗癫痫:一个有效性、安全性和经济概况的证据图
Authors Yi ZM, Wen C, Cai T, Xu L, Zhong XL, Zhan SY, Zhai SD
Received 30 July 2018
Accepted for publication 21 November 2018
Published 17 December 2018 Volume 2019:15 Pages 1—19
DOI https://doi.org/10.2147/NDT.S181886
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Objective: To
evaluate the efficacy, safety and economics of levetiracetam (LEV) for
epilepsy.
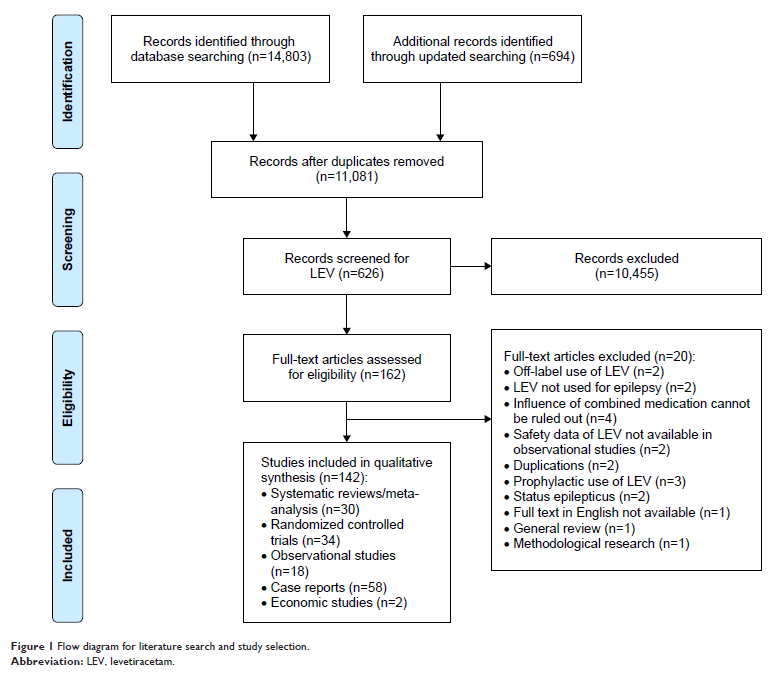
Materials and methods: PubMed,
Scopus, the Cochrane Library, OpenGrey.eu and ClinicalTrials.gov were
searched for systematic reviews (SRs), meta-analyses, randomized controlled
trials (RCTs), observational studies, case reports and economic studies
published from January 2007 to April 2018. We used a bubble plot to graphically
display information of included studies and conducted meta-analyses to
quantitatively synthesize the evidence.
Results: A total
of 14,803 records were obtained. We included 30 SRs/meta-analyses, 34 RCTs, 18
observational studies, 58 case reports and 2 economic studies after the
screening process. The included SRs enrolled patients with pediatric epilepsy,
epilepsy in pregnancy, focal epilepsy, generalized epilepsy and refractory
focal epilepsy. Meta-analysis of the included RCTs indicated that LEV was as
effective as carbamazepine (CBZ; treatment for 6 months: 58.9% vs 64.8%,
OR=0.76, 95% CI: 0.50–1.16; 12 months: 54.9% vs 55.5%, OR=1.24, 95% CI:
0.79–1.93), oxcarbazepine (57.7% vs 59.8%, OR=1.34, 95% CI: 0.34–5.23),
phenobarbital (50.0% vs 50.9%, OR=1.20, 95% CI: 0.51–2.82) and lamotrigine
(LTG; 61.5% vs 57.7%, OR=1.22, 95% CI: 0.90–1.66). SRs and observational
studies indicated a low malformation rate and intrauterine death rate for
pregnant women, as well as low risk of cognitive side effects. But psychiatric
and behavioral side effects could not be ruled out. LEV decreased
discontinuation due to adverse events compared with CBZ (OR=0.52, 95% CI:
0.41–0.65), while no difference was found when LEV was compared with placebo
and LTG. Two cost-effectiveness evaluations for refractory epilepsy with
decision-tree model showed US$ 76.18 per seizure-free day gained in Canada and
US$ 44 per seizure-free day gained in Korea.
Conclusion: LEV is as
effective as CBZ, oxcarbazepine, phenobarbital and LTG and has an advantage for
pregnant women and in cognitive functions. Limited evidence supports its
cost-effectiveness.
Registered number: PROSPERO
(No CRD 42017069367).
Keywords: seizure
freedom, responder rate, quality of life, malformations, neurological
development, psychiatric side effects, cost-effectiveness