110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

PD-L1 单克隆抗体 - 缀合的纳米颗粒可增强胃癌细胞中的药物递送水平和化学疗效
Authors Xu S, Cui F, Huang D, Zhang D, Zhu A, Sun X, Cao YM, Ding S, Wang Y, Gao E, Zhang F
Received 25 May 2018
Accepted for publication 29 October 2018
Published 18 December 2018 Volume 2019:14 Pages 17—32
DOI https://doi.org/10.2147/IJN.S175340
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Alexander Kharlamov
Peer reviewer comments 6
Editor who approved publication: Dr Lei Yang
Background: Docetaxel
(DOC) is widely used as a chemotherapy drug for various tumors, including
gastric cancer (GC), but the clinical application of DOC has been limited due
to the hydrophobicity of the drug. We aimed to formulate a multifunctional
nanoparticle (NP) system to reduce the side effects of the chemotherapy agent,
to promote synergistic therapeutic effects, and to achieve targeted delivery of
the therapy.
Methods: The
polyethylene glycol-poly(ε-caprolactone) NPs (PEG-PCL NPs) were prepared by a
ring opening copolymerization technique and were then conjugated with a
programmed death-ligand 1 (PD-L1) monoclonal antibody (mAb). The effects of the
surface coating on particle size, size distribution, zeta potential, drug
encapsulation efficiency, loading capacity, and the drug release kinetics were
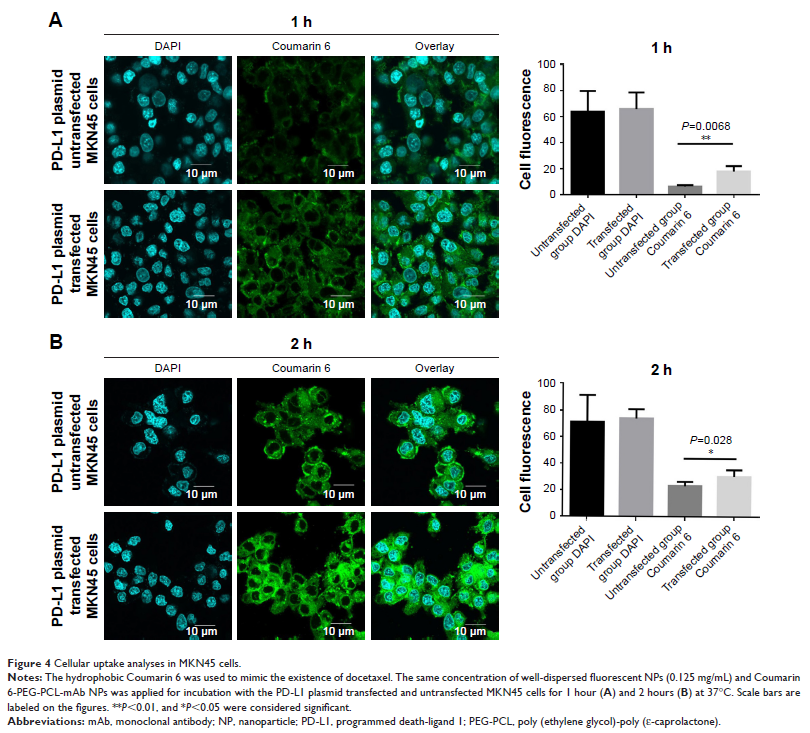
investigated. By using a panel of PD-L1-expressing human GC cell lines and
PD-L1-overexpressing cells, we studied cellular uptake, cytotoxic effects, and
cellular apoptosis in the presence of PD-L1 mAb-conjugated NPs.
Results: The
characterization of the structure and biological functions of DOC-PEG-PCL-mAb
NPs was investigated in vitro. X-ray photoelectron spectroscopy validated the
presence of the PD-L1 mAbs on the NP surface. The cellular uptake analysis
showed that the antibody-conjugated NPs achieved significantly higher cellular
uptake. The results of an in vitro cytotoxicity experiment on three GC lines
further proved the targeting effects of the antibody conjugation. In addition,
we found that the DOC-PEG-PCL-mAb NPs induced cell apoptosis and enhanced G2-M
arrest in cancer cells, indicating the inhibition of microtubule synthesis.
When compared with the control groups, DOC-PEG-PCL-mAb NPs are more effective
in inhibiting PD-L1 expression in GC cells.
Conclusion: Our
results reported here highlight the biological and clinical potential of
DOC-PEG-PCL-mAb NPs using PD-L1 mAbs in GC treatment.
Keywords: DOC,
gastric carcinoma, PD-L1 monoclonal antibody, nanomedicine, drug delivery