110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

含铂药物新辅助化疗对妊娠中晚期宫颈癌女性的疗效:一个更新的系统回顾和综合分析
Authors Song Y, Liu Y, Lin M, Sheng B, Zhu X
Received 9 September 2018
Accepted for publication 8 November 2018
Published 19 December 2018 Volume 2019:13 Pages 79—102
DOI https://doi.org/10.2147/DDDT.S186966
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 4
Editor who approved publication: Dr Tuo Deng
Purpose: To evaluate the
efficacy and safety of neoadjuvant platinum-based chemotherapy during pregnancy
in women with cervical cancer.
Methods: The
PubMed, Embase, and Cochrane Library databases were fully searched to find
eligible studies regarding platinum use during pregnancy in women with cervical
cancer from January 1980 to September 2018. Data were extracted from the
selected studies independently by two authors. Descriptive statistics were
calculated for categorical data (frequency and percentage) and numeration data
(mean and SD for normally distributed data and median and range for abnormally
distributed data). Survival analyses were performed using Kaplan–Meier survival
curves and log-rank tests to estimate overall survival and progression-free
survival for all patients.
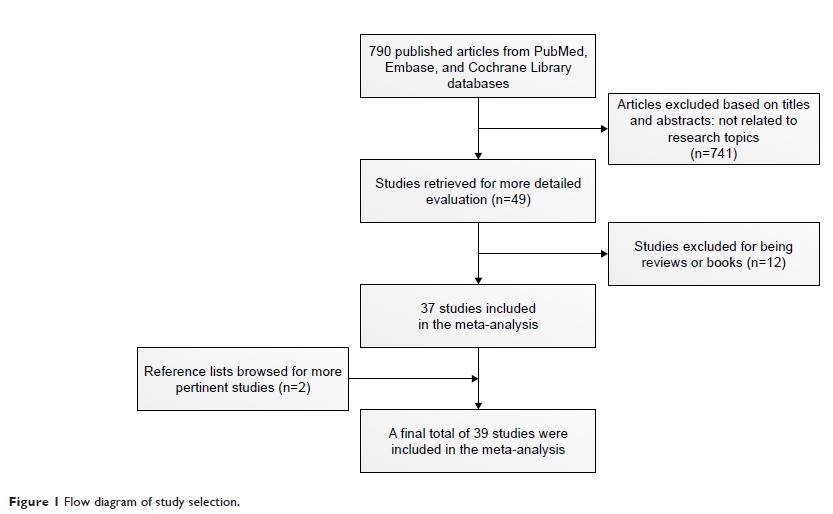
Results: A
total of 39 studies including 88 cervical cancer patients with platinum
administration during pregnancy were selected in this meta-analysis, and 64
women provided International Federation of Gynecology and Obstetrics stage
information. Among the latter, 56 of 64 (87.5%) were diagnosed with early
stages (I and IIA) and the remaining 8 of 64 (12.5%) had advanced stages (IIB,
III, and IV). In relation to cisplatin, 86 pregnant women were identified,
whereas only 2 pregnant women with carboplatin application were retrieved.
Overall, 88 newborns were delivered from 84 pregnancies, including two sets of
twins and one set of triplets, among which 71 neonates (71 of 88, 80.7%) were
completely healthy at birth. All children were healthy at the end of follow-up
(median 17 months, range 0–149.5 months), except one who was diagnosed with
retroperitoneal embryonal rhabdomyosarcoma at 5 years old and one who had acute
myeloid leukemia at 22 months of age. At the end of follow-up (range 4.75–156
months), 16 of 81 (19.8%) patients were diagnosed with recurrence of cervical
cancer, and 11 (90%) of those died because of cancer relapse. Neither median
overall survival nor median progression-free survival were reached.
Conclusion: Our
results demonstrated that neoadjuvant platinum-based chemotherapy could be a
favorable choice for the management of patients with cervical cancer during the
second and third trimesters. To reduce the side effects of chemotherapy,
cisplatin might be good to use as monotherapy in these patients.
Keywords: platinum,
neoadjuvant chemotherapy, pregnancy, cervical cancer, meta-analysis