110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

The number needed to treat and relevant between-trial comparisons of competing interventions
Authors Jansen JP, Khalid JM, Smyth MD, Patel H
Received 19 July 2018
Accepted for publication 9 November 2018
Published 14 December 2018 Volume 2018:10 Pages 865—871
DOI https://doi.org/10.2147/CEOR.S180491
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Lorenzo Colombo
Abstract: The
number needed to treat (NNT) is considered an intuitive as well as popular
effect measure. The aims of this review were to 1) explain why we cannot
compare trial-specific NNT estimates for the competing treatments evaluated in
different randomized controlled trials (RCTs) and 2) outline the principles of
how relative treatment effects of different trials can be compared and results
can be presented as NNT, without violating the principles of valid
between-trial comparisons. Our premise is that ratio measures for relative treatment
effects of response outcomes are less prone to effect modification than
absolute difference measures of response outcomes. Accordingly, any
between-trial comparisons of the efficacy of competing interventions using the
study-specific ORs are less likely to be invalid or biased than comparisons
based on the study-specific NNT estimates. However, treatment-specific ORs
obtained from a meta-analysis or taken directly from an individual study can be
transformed into consistent treatment-specific NNT estimates that allow for
credible comparisons of treatments when these ratio measures are applied to the
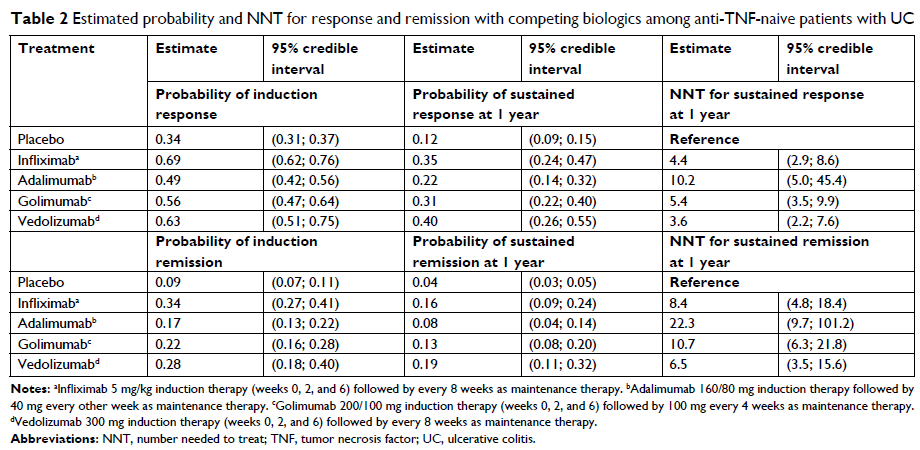
same reference response estimate. The theoretical discussion is illustrated
with a relevant indirect comparison of biologics for the treatment of ulcerative
colitis. Between-trial comparisons directly based on the NNT of individual
trials may result in erroneous conclusions and should be avoided.
Treatment-specific NNT estimates need to be based on the same probability of
response with the common reference treatment against which the interventions
are compared.
Keywords: biologics,
indirect treatment comparison, network meta-analysis, treatment outcomes,
ulcerative colitis