110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

以 SN38 为基础的新型衍生物脂质体作为抗癌前药:一项体内和体外研究
Authors Wu C, Zhang Y, Yang D, Zhang J, Ma J, Cheng D, Chen J, Deng L
Received 18 September 2018
Accepted for publication 29 November 2018
Published 20 December 2018 Volume 2019:14 Pages 75—85
DOI https://doi.org/10.2147/IJN.S187906
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Alexander Kharlamov
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
Background: Many novel drug
delivery systems have been extensively studied to exploit the full therapeutic
potential of SN38, which is one of the most potent antitumor analogs of
camptothecins (CPTs), whose clinical application is seriously hindered by poor
water solubility, low plasmatic stability, and severe toxicity, but results are
always unsatisfactory.
Methods: In this study,
combining the advantages of prodrug and nanotechnology, a lipophilic prodrug of
SN38, SN38-PA, was developed by conjugating palmitic acid to SN38 via ester
bond at C10 position, and then the lipophilic prodrug was
encapsulated into a long-circulating liposomal carrier by film dispersion
method.
Results: The SN38-PA
liposomes were characterized as follows: an average particle size of 80.13 nm,
an average zeta potential of -33.53 mv, and the entrapment efficiency of
99%. Compared with CPT-11, SN38-PA liposome was more stable in close lactone
form, more efficient in conversion rate to SN38, and more potent in cytotoxicity
against tumor cells. Pharmacokinetic study showed that SN38-PA liposome had
significantly enhanced plasma half-life (t1/2) value of SN38
and increased area under the curve (AUC) of SN38, which was 7.5-fold higher
than that of CPT-11. Biodistribution study showed that SN38-PA liposome had
more active metabolite SN38 in each tissue. Finally, the pharmacodynamic study
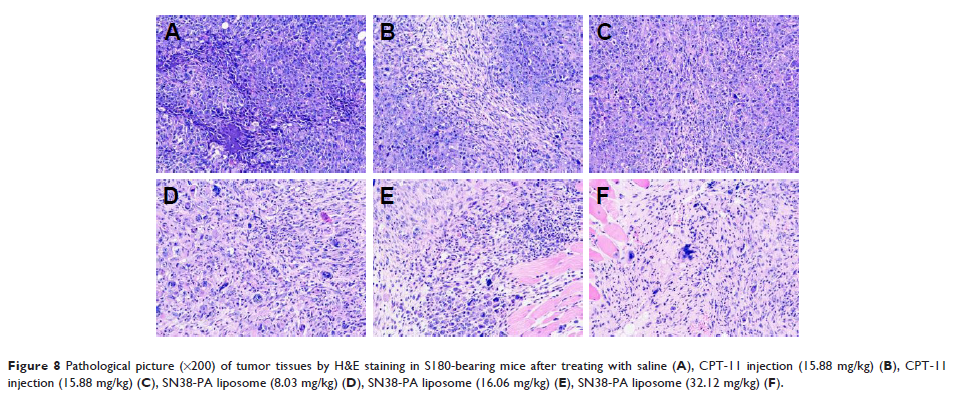
showed that SN38-PA liposome had higher antitumor effect with the antitumor
inhibition rate of 1.61 times than that of CPT-11.
Conclusion: These
encouraging data merit further investigation on this novel SN38-PA liposome.
Keywords: SN38,
lipophilic prodrug, long-circulating liposome, pharmacokinetics,
biodistribution, pharmacodynamics, CPT-11