110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

miR-125a 通过靶向胃癌细胞中的 STAT3 来抑制细胞迁移和侵袭
Authors Yang L, Zhang S, Guo K, Huang H, Qi S, Yao J, Zhang Z
Received 16 March 2018
Accepted for publication 18 November 2018
Published 24 December 2018 Volume 2019:12 Pages 205—215
DOI https://doi.org/10.2147/OTT.S168454
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 5
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Background: Recently, many microRNAs have been found
to be involved in the cancer progression including miR-125a. However, the
underlying mechanisms of miR-125a in gastric cancer (GC) remain to be
completely elucidated.
Objective: The
study was to investigate the functional role of miR-125a and the expression relevance
of signal transducer and activator of transcription 3 (STAT3) and hyaluronan
synthase 1 (HAS1).
Method: CCK-8
assay, scratch wound healing and transwell assay were conducted to identify the
functional role of miR-125a in GC. In addition, using bioinformatics analysis,
the target regulation relationship was found in STAT3 and miR-125a. To confirm
the relationship, luciferase reporter assay was performed. More importantly,
quantitative polymerase chain reaction and western blot assay were carried out
to determine the association among miR-125a, STAT3 and HAS1 in GC cells.
Results: Overexpressed
miR-125a inhibited the migration and invasion of GC cells through scratch wound
healing and transwell assay, and its knockdown displayed adverse effects, but the
viability of GC cells did not show significant difference using CCK-8 assay. In
addition, we identified that the knockdown of STAT3 or HAS1 remarkably
suppressed the migration and invasion abilities of GC cells. Using
bioinformatics analysis, miRTar, in particular, indicated that the
3'-untranslated region of STAT3 binds to miR-125a with a high score.
Subsequently, we also verified that STAT3 was a target of miR-125a via
luciferase reporter assay. Furthermore, we found that upregulated miR-125a
expression could conspicuously constrain STAT3 expression at both protein and
mRNA levels in MKN45 and NCI-N87 cells using quantitative polymerase chain
reaction and Western blot assay, but no significant difference had been found
in SGC 7901 cells. To further identify the regulatory relationship between
miR-125a and STAT3, downregulation of miR-125a in MKN45 and NCI-N87 cells was
carried out, which showed that the protein and mRNA expression levels of STAT3
were declined in two cell lines. Finally, we observed that upregulated miR-125a
could lead to the decrease of HAS1 at protein and mRNA levels, whereas its
knockdown revealed opposite effects. Meanwhile, we noticed that overexpression
of STAT3 could induce the escalation of HAS1 at protein and mRNA expression levels
and its knockdown exhibited the adverse outcomes.
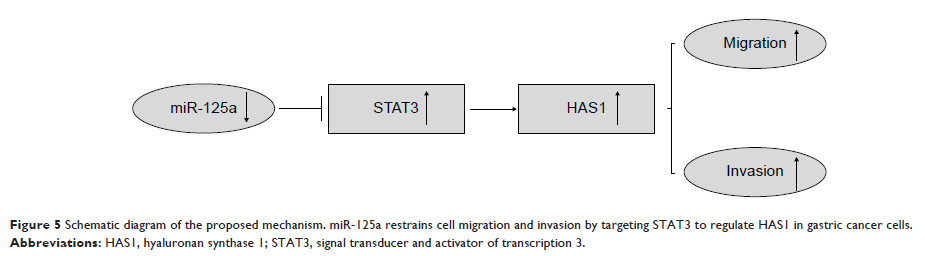
Conclusion: These
findings indicated that miR-125a may control the HAS1 expression in GC
progression by targeting STAT3, which is likely to facilitate a better
understanding of the regulation mechanisms of miR-125a in GC.
Keywords: miR-125a,
STAT3, HAS1, gastric cancer