110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

转为阿那曲唑和戈舍瑞林治疗与继续使用他莫昔芬辅助治疗绝经前早期乳腺癌的对比:随机试验的初步结果
Authors Li J, Liu G, Ji Y, Yan X, Pang D, Jiang Z, Chen D, Zhang B, Xu B, Shao Z
Received 12 August 2018
Accepted for publication 19 November 2018
Published 27 December 2018 Volume 2019:11 Pages 299—307
DOI https://doi.org/10.2147/CMAR.S183672
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Purpose: To assess
the efficacy, safety, and quality-of-life impact of switching adjuvant
treatment in hormone receptor-positive primary breast cancer patients who are
still premenopausal after 2–3 years of tamoxifen therapy to anastrozole plus goserelin
as compared with continuing tamoxifen over a total period of 5 years.
Patients and methods: Hormone
receptor-positive, premenopausal, lymph node-positive, or tumor size ≥4 cm
breast cancer patients who had received tamoxifen for 2–3 years were randomly
assigned to continue tamoxifen treatment (TAM group) or switch to adjuvant
anastrozole plus goserelin (ADD group) and continue treatment for another 2–3
years (total treatment duration 5 years). Endpoints evaluated were adverse
events (AEs), changes in bone mineral density, quality of life, and
disease-free survival-related events.
Results: A total
of 62 patients (33 in the ADD group and 29 in the TAM group) were evaluated.
Grade 3–4 drug-related AEs occurred in five patients (15.2%) in the ADD group
vs none in the TAM group. In the ADD group, arthralgias were the most common
AEs (5/33 patients; 15.2%), and three patients in this group were discontinued
because of AEs. Treatment was temporarily suspended due to AEs in three
patients (9.1%) in the ADD group and one patient (3.4%) in the TAM group.
Compared with continuing TAM therapy, switching to anastrozole plus goserelin
did not result in any worsening of bone mineral density or quality of life.
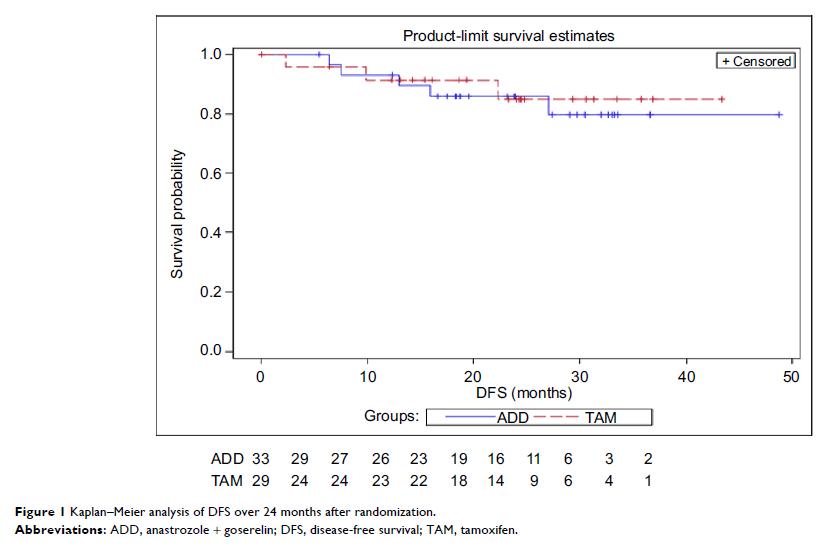
During a median follow-up of 34 months, five patients (15.2%) in the ADD group
had disease-free survival events vs four patients (13.8%) in the TAM group.
Conclusion: For
early-stage breast cancer patients who remain premenopausal following 2–3 years
of adjuvant tamoxifen therapy, switching to anastrozole plus goserelin therapy
was safe with tolerable adverse effects. However, it did not show superior
efficacy compared to remaining on tamoxifen treatment.
Trial Registration: ClinicalTrials.gov
(identifier NCT01352091).
Keywords: breast
cancer, adjuvant therapy, anastrozole, tamoxifen, GnRH analogs, aromatase
inhibitors