110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Effects of baseline symptom burden on treatment response in COPD
Authors Martinez FJ, Abrahams RA, Ferguson GT, Bjermer L, Grönke L, Voß F, Singh D
Received 12 July 2018
Accepted for publication 18 November 2018
Published 4 January 2019 Volume 2019:14 Pages 181—194
DOI https://doi.org/10.2147/COPD.S179912
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Rationale: In symptomatic patients with COPD, the
decision whether to initiate maintenance treatment with a single agent or a
combination of long-acting bronchodilators remains unclear.
Objective: To
investigate whether baseline symptomatic status influences response to
tiotropium/olodaterol treatment.
Materials and methods: Post hoc analysis of the randomized OTEMTO® studies (NCT01964352; NCT02006732), in which
patients with moderate-to-severe COPD received placebo, tiotropium 5 µg,
tiotropium/olodaterol 2.5/5 µg, or tiotropium/olodaterol 5/5 µg once
daily for 12 weeks via the Respimat® inhaler
(Boehringer Ingelheim, Ingelheim am Rhein, Germany). Impact of baseline
symptomatic status (modified Medical Research Council [mMRC] score) on response
to treatment with tiotropium/olodaterol 5/5 µg, tiotropium 5 µg, or
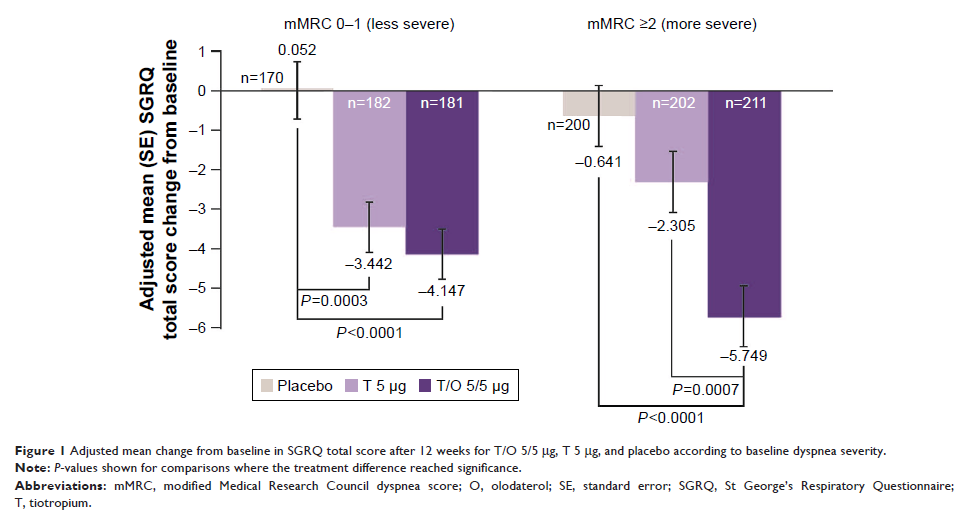
placebo at Week 12 was assessed by St George’s Respiratory Questionnaire (SGRQ)
total score and response rate, transition dyspnea index (TDI) focal score and
response rate, and trough forced expiratory volume in 1 second response.
Results: Tiotropium/olodaterol
improved SGRQ total scores and response rates compared with placebo and
tiotropium for patients with baseline mMRC scores 0–1 and ≥2. For
tiotropium/olodaterol vs tiotropium, greater improvements were observed for
patients with mMRC ≥2 (SGRQ score adjusted mean treatment difference -3.44 [95%
CI: -5.43, -1.46]; P =0.0007; SGRQ response rate ORs 2.09 [95% CI: 1.41,
3.10]; P =0.0002).
Dyspnea, measured by TDI score, was consistently improved with
tiotropium/olodaterol vs placebo for patients with mMRC scores 0–1 and ≥2
(adjusted mean treatment difference 1.63 [95% CI: 1.06, 2.20]; P <0.0001 and
1.60 [95% CI: 1.09, 2.10]; P <0.0001, respectively). In patients with mMRC
scores 0–1 and ≥2, tiotropium/olodaterol consistently improved TDI response
rate and lung function vs placebo and tiotropium.
Conclusions: Patients
with COPD with more severe baseline dyspnea appear to derive greater health
status benefit with tiotropium/olodaterol compared with tiotropium alone.
Keywords: tiotropium,
olodaterol, COPD