110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

在 BRAF 突变转移性结直肠癌治疗中使用氟尿嘧啶、伊立替康与 BRAFV600E 和 EGFR 抑制剂的完全反应:一份病例报告
Authors Wang Z, Dai WP, Zang YS
Received 19 July 2018
Accepted for publication 5 December 2018
Published 8 January 2019 Volume 2019:12 Pages 443—447
DOI https://doi.org/10.2147/OTT.S180845
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Background: Patients
with BRAF (v-Raf murine sarcoma viral oncogene homolog B) V600E-mutated
metastatic colorectal cancer (mCRC) have a poor prognosis. The Southwest
Oncology Group (SWOG) 1406 study evaluated the efficacy of vemurafenib in
combination with irinotecan and cetuximab for simultaneous inhibition of
epidermal growth factor receptor (EGFR) and BRAF in patients with BRAFV600E-mutated mCRC.
Although the combination achieved higher progression-free survival (PFS) and
disease control rates (DCRs), there was no complete response (CR) for the drug
combination. In this case report, we report the complete recession of
metastasis in a patient treated with irinotecan, cetuximab, vemurafenib, and
5-fluorouracil.
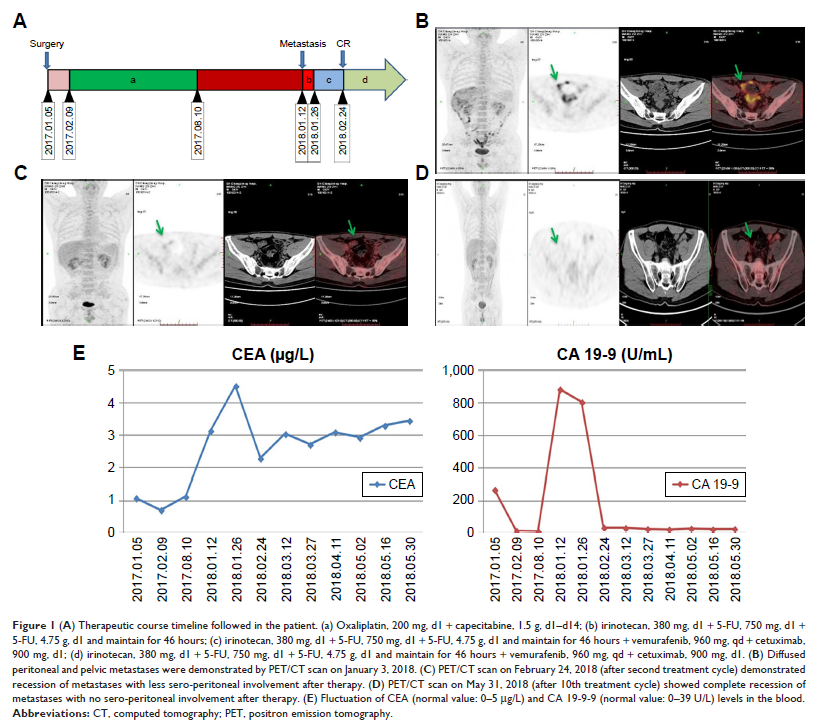
Case presentation: A
44-year-old male patient with hepatitis B was diagnosed with right-sided colon
adenocarcinoma. He was treated with capecitabine plus oxaliplatin as postoperative
adjuvant chemotherapy for eight cycles with a disease-free survival (DFS) of 1
year before the emergence of peritoneal and pelvic metastases. BRAFV600E mutation
was positive and chemotherapy included 12 courses of 5-fluorouracil,
vemurafenib, irinotecan, and cetuximab. Complete response with recession of
metastases was observed.
Conclusion: The combination
of fluorouracil and irinotecan with a BRAFV600E and EGFR
inhibitor may have synergistic action, leading to recession of secondary
metastases in patients with BRAFV600E-mutated
colorectal cancer.
Keywords: mCRC,
BRAFV600E mutation, fluorouracil, vemurafenib,
irinotecan, cetuximab