110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

TiO2 纳米管通过 ERK1/2 和 PI3K/AKT 通路调节巨噬细胞 M2 极化并增加 VEGF 的巨噬细胞分泌以加速内皮化
Authors Xu WC, Dong X, Ding JL, Liu JC, Xu JJ, Tang YH, Yi YP, Lu C, Yang W, Yang JS, Gong Y, Zhou JL
Received 22 September 2018
Accepted for publication 10 December 2018
Published 10 January 2019 Volume 2019:14 Pages 441—455
DOI https://doi.org/10.2147/IJN.S188439
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Govarthanan Muthusamy
Peer reviewer comments 4
Editor who approved publication: Dr Mian Wang
Background: Macrophages
play important roles in the immune response to, and successful implantation of,
biomaterials. Titanium nanotubes are considered promising heart valve stent
materials owing to their effects on modulation of macrophage behavior. However,
the effects of nanotube-regulated macrophages on endothelial cells, which are
essential for stent endothelialization, are unknown. Therefore, in this study
we evaluated the inflammatory responses of endothelial cells to titanium
nanotubes prepared at different voltages.
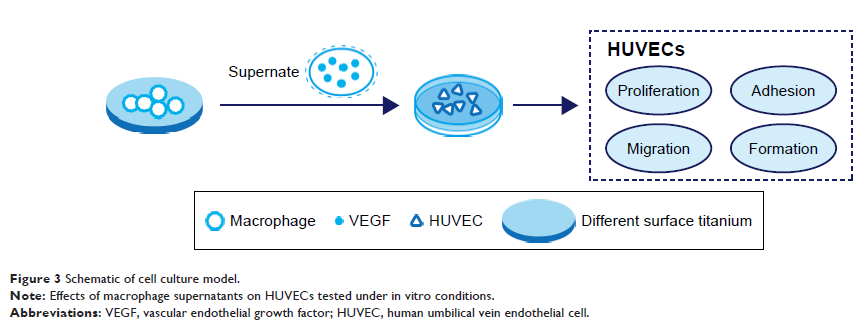
Methods and results: In this study we used three different voltages (20, 40,
and 60 V) to produce titania nanotubes with three different diameters by anodic
oxidation. The state of macrophages on the samples was assessed, and the
supernatants were collected as conditioned media (CM) to stimulate human
umbilical vein endothelial cells (HUVECs), with pure titanium as a control
group. The results indicated that titanium dioxide (TiO2) nanotubes
induced macrophage polarization toward the anti-inflammatory M2 state and
increased the expression of arginase-1, mannose receptor, and interleukin 10.
Further mechanistic analysis revealed that M2 macrophage polarization
controlled by the TiO2nanotube surface activated the phosphatidylinositol 3-kinase/AKT
and extracellular signal-regulated kinase 1/2 pathways through release of
vascular endothelial growth factor to influence endothelialization.
Conclusion: Our
findings expanded our understanding of the complex influence of nanotubes in
implants and the macrophage inflammatory response. Furthermore, CM generated
from culture on the TiO2 nanotube surface may represent an integrated research
model for studying the interactions of two different cell types and may be a
promising approach for accelerating stent endothelialization through
immunoregulation.
Keywords: TiO2 nanotubes,
axitinib, stent implant, endothelial cells, conditioned medium