110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

装载伊达比星的甲氧基聚乙二醇-b -聚(L-丙交酯-乙交酯)用于增强细胞摄取和促进抗白血病活性
Authors Liang B, Li N, Zhang S, Qi A, Feng J, Jing W, Shi C, Ma Z, Gao S
Received 7 October 2018
Accepted for publication 10 December 2018
Published 11 January 2019 Volume 2019:14 Pages 543—556
DOI https://doi.org/10.2147/IJN.S190027
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Govarthanan Muthusamy
Peer reviewer comments 2
Editor who approved publication: Dr Mian Wang
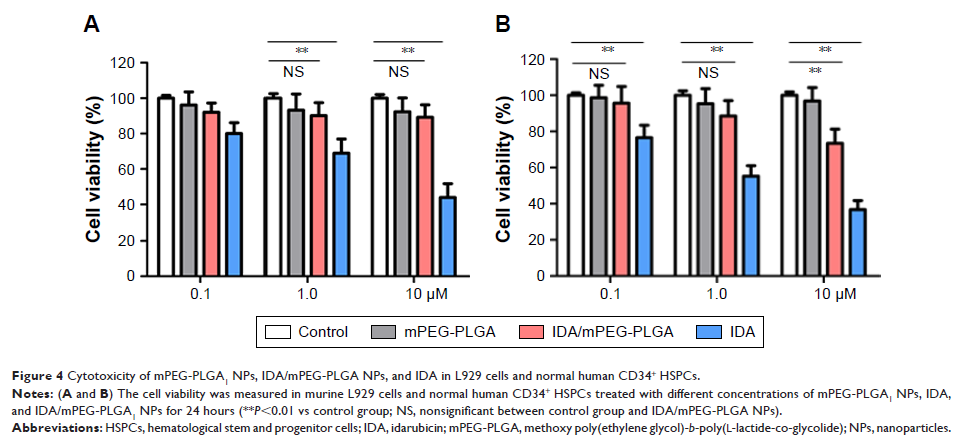
Purpose: Nanoparticle
(NP)-based drug delivery approaches have tremendous potential for enhancing
treatment efficacy and decreasing doses of chemotherapeutics. Idarubicin (IDA)
is one of the most common chemotherapeutic drugs used in the treatment of acute
myeloid leukemia (AML). However, severe side effects and drug resistance
markedly limit the application of IDA.
Methods: In this
study, we encapsulated IDA in polymeric NPs and validated their antileukemia
activity in vitro and in vivo.
Results: NPs with
an average diameter of 84 nm was assembled from a methoxy poly(ethylene
glycol)-b -poly(l-lactide-co-glycolide)
(mPEG-PLGA). After loading of IDA, IDA-loaded mPEG-PLGA NPs (IDA/mPEG-PLGA NPs)
were formed. The in vitro release data showed that the IDA/mPEG-PLGA NPs have
excellent sustained release property. IDA/mPEG-PLGA NPs had exhibited the lower
IC50 than pure IDA. Moreover, IDA/mPEG-PLGA
NPs in the same concentration substantially induced apoptosis than did pure
IDA. Most importantly, IDA/MPEG-PLGA NPs significantly decreased the
infiltration of leukemia blasts and improved the overall survival of MLL-AF9-induced
murine leukemia compared with free IDA. However, the blank NPs were nontoxic to
normal cultured cells in vitro, suggesting that NPs were the safe carrier.
Conclusion: Our data
suggest that IDA/mPEG-PLGA NPs might be a suitable carrier to encapsulate IDA.
Low dose of IDA/mPEG-PLGA NPs can be used as a conventional dosage for
antileukemia therapy to reduce side effect and improve survival.
Keywords: idarubicin,
mPEG-PLGA, acute myeloid leukemia, nanoparticles