110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

NR1H3 在子宫内膜癌中的表达及其对 Ishikawa 细胞增殖的影响
Authors Fang F, Li D, Zhao L, Li Y, Zhang T, Cui B
Received 17 July 2018
Accepted for publication 5 November 2018
Published 18 January 2019 Volume 2019:12 Pages 685—697
DOI https://doi.org/10.2147/OTT.S180534
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev Srivastava
Purpose: Our study
aimed to investigate the expression of NR1H3 in endometrial carcinoma, its
effect on the proliferation of endometrial carcinoma cells in vitro, and
the underlying mechanism of this effect.
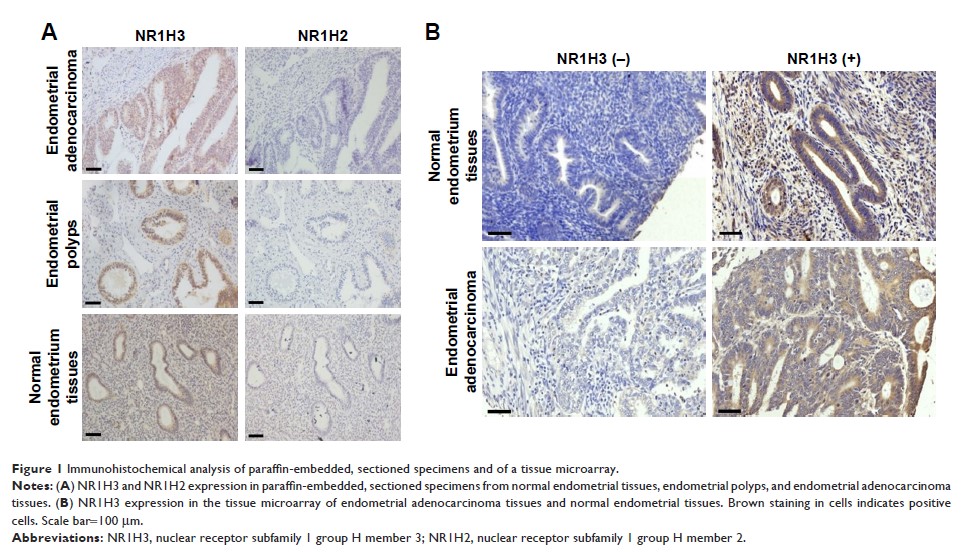
Materials and methods: Immunohistochemistry
of paraffin-embedded, sectioned specimens and of a tissue microarray was
conducted to estimate the expression of NR1H3 (liver X receptors α: LXRα) and
NR1H2 (liver X receptors β: LXRβ) in endometrial carcinoma tissues. The
subcellular localization of NR1H3 in the endometrial carcinoma cell line
Ishikawa was determined by immunofluorescence. An agonist of NR1H3, TO901317,
was then administered to activate the expression of NR1H3, and cell viability
and cell-cycle progression were investigated through MTT and flow cytometric
assays, respectively. The gene and protein expression levels of NR1H3, cyclin
D1 (CCND1), and cyclin E (CCNE) in cells pretreated with different
concentrations of TO901317 for different periods of time were also detected by
real-time RT-PCR and Western blot, respectively.
Results: The
results showed that, in contrast to NR1H2, which was expressed at low levels in
endometrial tissues, NR1H3 was upregulated in endometrial adenocarcinoma
tissues compared to levels in normal endometrial tissues and endometrial
polyps. Moreover, NR1H3 was mainly expressed in the cytoplasm of Ishikawa
cells. TO901317 significantly decreased cell viability and arrested the cell
cycle in Ishikawa cells in a dose- and time-dependent manner. Furthermore, the
administration of TO901317 not only promoted the expression of NR1H3 but also
inhibited the expression of CCND1 and CCNE in Ishikawa cells.
Conclusion: We
demonstrated that NR1H3 is upregulated in endometrial adenocarcinoma and that
it inhibits cell viability by inhibiting the expression of CCND1 and CCNE in
endometrial carcinoma cells. Our study indicates that NR1H3 may play a role in
the development of endometrial cancer and may emerge as a promising therapeutic
target.
Keywords: liver X
receptor, CCND1, endometrial carcinoma, cell proliferation, cell cycle