110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

纳武单抗治疗转移性胆道癌的疗效和安全性
Authors Gou M, Zhang Y, Si H, Dai G
Received 22 November 2018
Accepted for publication 1 January 2019
Published 25 January 2019 Volume 2019:12 Pages 861—867
DOI https://doi.org/10.2147/OTT.S195537
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev Srivastava
Objective: PD-1
inhibitors have improved efficacy in many cancers. There are currently no
reports of the use of PD-1 inhibitors, such as nivolumab, for metastatic
biliary tract cancer (mBTC). This study reviewed the efficacy and safety of
nivolumab for mBTC with the aim of exploring ways to improve efficacy and
survival.
Methods: Thirty
patients with mBTC were voluntarily treated with nivolumab at the PLA General
Hospital. Nivolumab 3 mg/kg was administered. Progression-free survival (PFS)
and overall survival were evaluated by Kaplan–Meier and univariate and
multivariate analyses were carried out for clinical characteristics. Objective
response rate (ORR), disease control rate (DCR), and treatment-related adverse
events (AEs) were also evaluated.
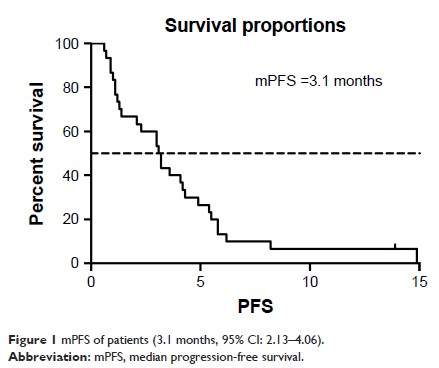
Results: The
median treatment cycle is four cycles. One case was complete response, 5 cases
partial response, 12 cases stable, and 12 cases progression. ORR was 20%, DCR
was 60%, and PFS was 3.1 months (95% CI: 2.13–4.06). The AEs of nivolumab
monotherapy were fatigue (three cases), fever (two cases), hypothyroidism (one
case), skin reaction (one case), and liver injury (one case). Nivolumab
combined with chemotherapy related grade 1–2 hematologic toxicity were leukopenia
(five cases) and thrombocytopenia (two cases), and grade 3–4 were leukopenia
(three cases). Non-hematologic toxicity grade 1–2 were nausea and vomiting
(four cases), fatigue (four cases), fever (three cases), peripheral
neurotoxicity (three cases), and hypothyroidism (one case). Univariate analysis
showed that PFS of nivolumab combined with chemotherapy was statistically
significant compared with that of nivolumab monotherapy (4.1 vs 2.3
months, P =0.031).
Programmed death-ligand 1 (PD-L1) expression positively has no relationship
with better PFS in contrast with PD-L1 negatively (3.6 vs 3.0 months P >0.05).
Multivariate analysis show nivolumab combined with chemotherapy was only the
independent factor for longer PFS (HR: 0.432, P <0.05).
Conclusion: The safety
of nivolumab in mBTC is controllable. Further selection of superior populations
is needed to improve the efficacy of nivolumab in mBTC.
Keywords: metastatic
biliary tract cancer, nivolumab, PD-L1, PD-1