110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

基于双模式氧化铁纳米颗粒的硫化氢供体控释可保护心肌组织免受缺血 - 再灌注损伤
Authors Wang W, Liu H, Lu YT, Wang X, Zhang B, Cong S, Zhao Y, Ji M, Tao H, Wei L
Received 3 September 2018
Accepted for publication 30 November 2018
Published 30 January 2019 Volume 2019:14 Pages 875—888
DOI https://doi.org/10.2147/IJN.S186225
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Govarthanan Muthusamy
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Background: Hydrogen
sulfide (H2S) has shown promising therapeutic benefits in
reversing a variety of pathophysiological processes in cardiovascular system,
including myocardial ischemia–reperfusion (IR) injury. However, the achievement
of controlled and sustained release of H2S has been a
technical bottleneck that limits the clinical application of the gas molecule.
Methods: The
current study describes the development of mesoporous iron oxide nanoparticles
(MIONs) which were loaded with diallyl trisulfide (DATS), a H2S donor
compound, and calibrated by stimulated Raman scattering/transient absorption.
Results: The
synthesized MIONs were characterized with excellent mesoporosity and a narrow
size distribution, which enabled them to slow down the release of H2S to a suitable
rate and prolong the plateau period. The controlled-release feature of
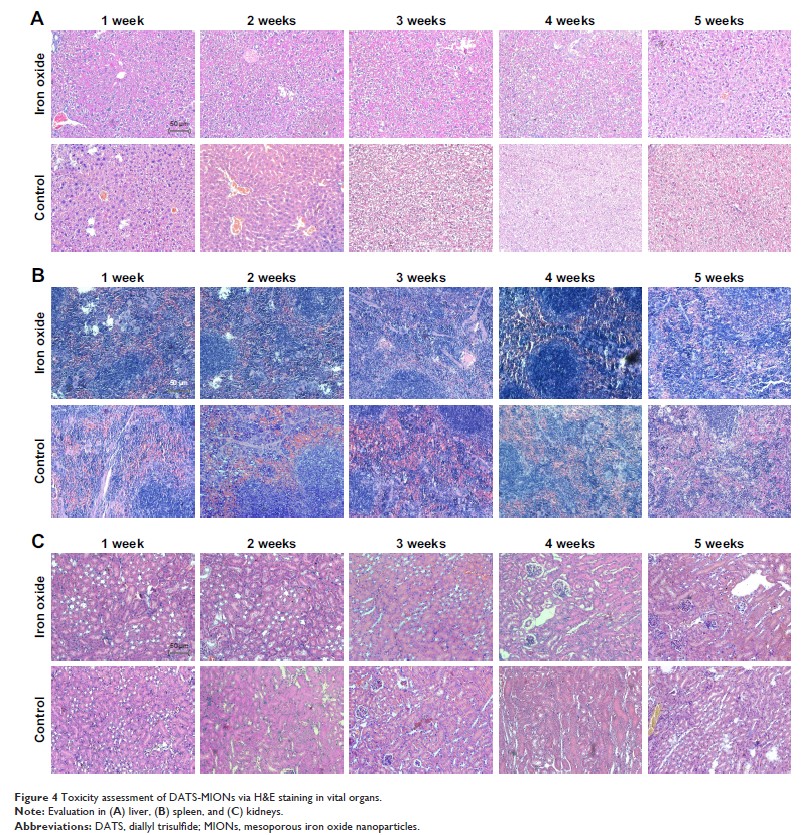
DATS-MIONs resulted in little adverse effect both in vitro and
in vivo, and their protective effect on the heart tissue that underwent IR
injury was observed in the mouse model of myocardial ischemia. The rapid
biodegradation of DATS-MIONs was induced by Kupffer cells, which were
specialized macrophages located in the liver and caused limited hepatic
metabolic burden.
Conclusion: The
sustained-release pattern and excellent biocompatibility make DATS-MIONs a
promising H2S donor for research and medical purposes.
Keywords: steady
release, porous structure, biocompatibility, biodegeneration