110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

癌症患者中与易普利姆玛联合纳武单抗治疗,及纳武单抗治疗相关联的免疫相关不良事件的风险
Authors Zhou S, Khanal S, Zhang H
Received 3 November 2018
Accepted for publication 28 December 2018
Published 31 January 2019 Volume 2019:15 Pages 211—221
DOI https://doi.org/10.2147/TCRM.S193338
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 2
Editor who approved publication: Professor Deyun Wang
Purpose: The aim
of this study was to evaluate the risk of immune-related adverse events (irAEs)
among cancer patients receiving nivolumab-plus-ipilimumab therapy and nivolumab
monotherapy.
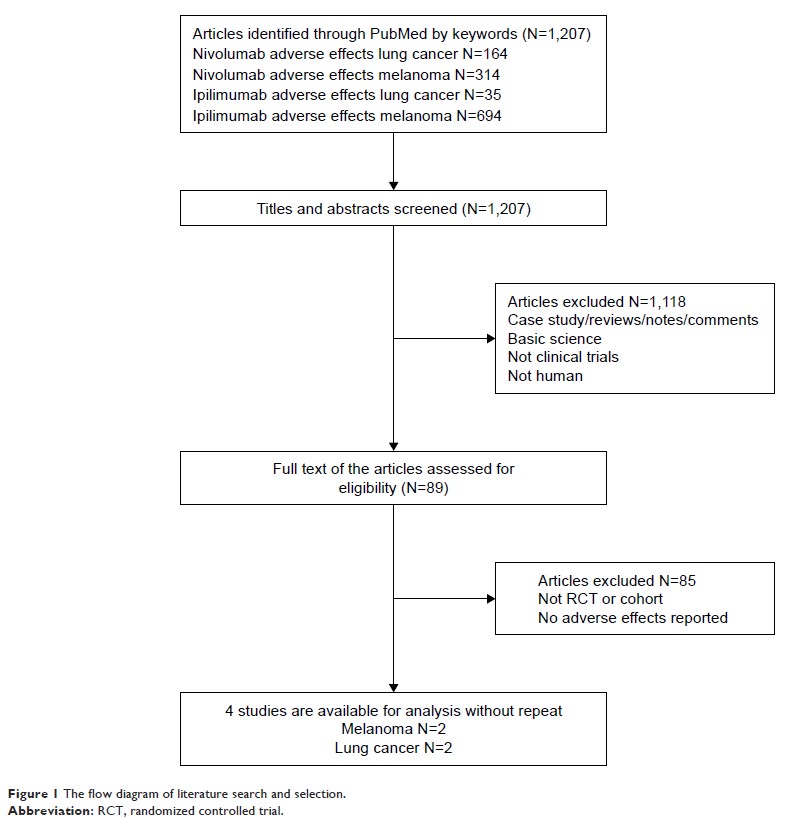
Patients and methods: PubMed
and Web of Science were searched for related studies from inception to June
2018. Eligible studies included randomized controlled trials comparing nivolumab-plus-ipilimumab
with nivolumab alone in cancer patients reporting on all-grade (grade 1–4) and
high-grade (grade 3/4) irAEs. Paired reviewers selected studies for inclusion
and extracted data. The odds risk and 95% CI were calculated.
Results: A total
of 2,946 patients from four studies were included in the meta-analysis. The
underlying malignancies included lung cancer (two trials) and melanoma (two
trials). Compared with nivolumab monotherapy, the nivolumab-plus-ipilimumab
therapy was associated with a significantly higher risk of all- and high-grade
irAEs such as pruritus, rash, diarrhea, colitis, alanine aminotransferase
elevation, and pneumonitis.
Conclusion: The
combination therapy of nivolumab and ipilimumab increased the incidence of
irAEs in patients with advanced cancer.
Keywords: immune-related
adverse events, immune checkpoint inhibitors, nivolumab, ipilimumab, lung
cancer, melanoma