111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

CMAB008 的物理化学表征和 I 期研究,它是由不同表达系统产生的英夫利昔单抗生物仿制药
Authors An Q, Zheng Y, Zhao Y, Liu T, Guo H, Zhang D, Qian W, Wang H, Guo Y, Hou S, Li J
Received 12 April 2018
Accepted for publication 31 July 2018
Published 12 March 2019 Volume 2019:13 Pages 791—805
DOI https://doi.org/10.2147/DDDT.S170913
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Lucy Goodman
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Background: Infliximab
(Remicade), a chimeric monoclonal antibody against human TNFα, will inevitably
face competition from biosimilar products, because of its effectiveness in
autoimmune diseases and rapidly increasing market demand. According to
guidelines for biosimilar development, the “biosimilar-expression system” may
differ from that of the innovator, but more appropriate studies should be
carried out to demonstrate the comparability between biosimilar and innovator.
CMAB008 is an infliximab biosimilar candidate developed by the State Key
Laboratory of Antibody Medicine and Targeted Therapy of China. Infliximab was
expressed in SP2/0 cells, while CMAB008 was produced in a CHO-expression
system.
Methods: In this
study, infliximab and CMAB008 were compared on physicochemical and biological
characterizations, including protein content, activity, physiochemical
integrity, impurities, additives, and immunogenicity.
Results: The
results showed that they were highly similar and comparable, except some
differences in glycosylation. As glycosylation profiles can influence
immunogenicity and occurrence of allergy or other adverse reactions of antibody
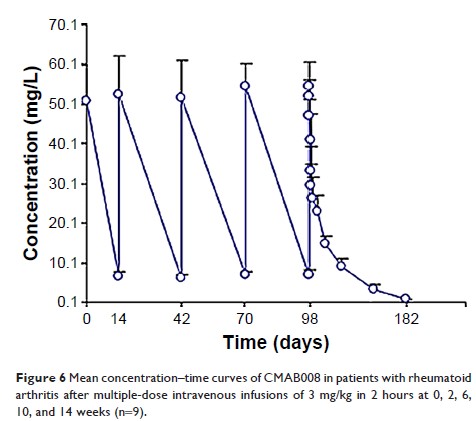
therapeutics, primary tolerability and pharmacokinetics of CMAB008 were
evaluated. In the phase I clinical trial, plasma concentration of CMAB008 and
antidrug antibodies were also measured using ELISA and bridging ELISA,
respectively. CMAB008 exhibited favorable clinical tolerability, no adverse
events in the 3 mg/kg single-dose group (recommended therapeutic dosage), and
no serious adverse events in the multiple-dose group. Also, no injection-site
reactions were observed in the experiment.
Conclusion: In
summary, CMAB008 might have the potential to be an effective drug compared with
infliximab.
Keywords: infliximab,
biosimilar, “biobetter”, CMAB008, immunogenicity