111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

通过整合生物信息学分析鉴定 CUMS 诱导抑郁的关键基因、途径和伏隔核中 miRNA/mRNA 调控网络
Authors Ma K, Zhang HX, Wei GH, Dong ZF, Zhao HJ, Han XC, Song XB, Zhang HL, Zong X, Baloch Z, Wang SJ
Received 3 January 2019
Accepted for publication 10 February 2019
Published 14 March 2019 Volume 2019:15 Pages 685—700
DOI https://doi.org/10.2147/NDT.S200264
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 2
Editor who approved publication: Dr Yu-Ping Ning
Introduction: Major
depressive disorder (MDD) is a recurrent, devastating mental disorder, which
affects >350 million people worldwide, and exerts substantial public health
and financial costs to society. Thus, there is a significant need to discover
innovative therapeutics to treat depression efficiently. Stress-induced
dysfunction in the subtype of neuronal cells and the change of synaptic plasticity
and structural plasticity of nucleus accumbens (NAc) are implicated in
depression symptomology. However, the molecular and epigenetic mechanisms and
stresses to the NAc pathological changes in depression remain elusive.
Materials and methods: In this
study, treatment group mice were treated continually with the chronic
unpredictable mild stress (CUMS) until expression of depression-like behaviors
were found. Depression was confirmed with sucrose preference,
novelty-suppressed feeding, forced swimming, and tail suspension tests. We
applied high-throughput RNA sequencing to assess microRNA expression and
transcriptional profiles in the NAc tissue from depression-like behaviors mice
and control mice. The regulatory network of miRNAs/mRNAs was constructed based
on the high-throughput RNA sequence and bioinformatics software predictions.
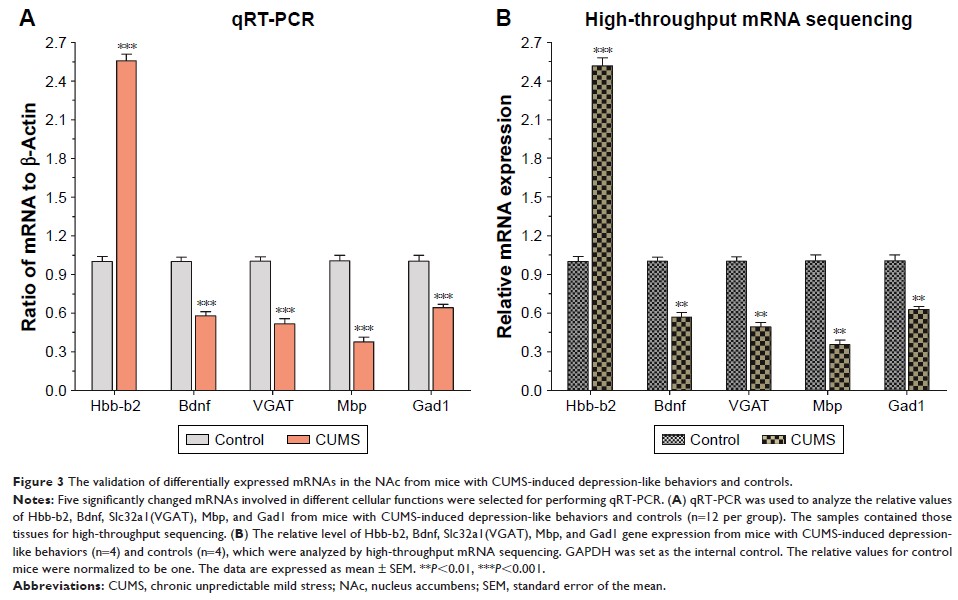
Results: A total
of 17 miRNAs and 10 mRNAs were significantly upregulated in the NAc of
CUMS-induced mice with depression-like behaviors, and 12 miRNAs and 29 mRNAs
were downregulated. A series of bioinformatics analyses showed that these
altered miRNAs predicted target mRNA and differentially expressed mRNAs were
significantly enriched in the MAPK signaling pathway, GABAergic synapse,
dopaminergic synapse, cytokine–cytokine receptor interaction, axon guidance,
regulation of autophagy, and so on. Furthermore, dual luciferase report assay
and qRT-PCR results validated the miRNA/mRNA regulatory network.
Conclusion: The
deteriorations of GABAergic synapses, dopaminergic synapses, neurotransmitter
synthesis, as well as autophagy-associated apoptotic pathway are associated
with the molecular pathological mechanism of CUMS-induced depression.
Keywords: stress,
depression, nucleus accumbens, miRNA, mRNA