111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

PF4V1 , miRNA-875-3p 靶标,抑制前列腺癌细胞增殖、迁移和侵袭,并作为潜在的预后生物标志物
Authors Li D, Hao X, Dong Y, Zhang M, Song Y
Received 17 September 2018
Accepted for publication 31 December 2018
Published 21 March 2019 Volume 2019:11 Pages 2299—2312
DOI https://doi.org/10.2147/CMAR.S187831
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 4
Editor who approved publication: Dr Rituraj Purohit
Background: PF4V1 is
a novel protein in inflammation, angiogenesis, and cancer. However, the
pathogenesis, underlying mechanisms, and the prognostic value of PF4V1 in
prostate cancer (PCa) are still unclear.
Materials and methods: The PF4V1
expression and relation with survival were analyzed based on a large sample
size in the Cancer Genome Atlas. In vitro, the overexpression of PF4V1 was
conducted in DU145 and LNCaP cells. Cell Counting Kit-8, colony formation,
wound healing, and Transwell® assays were preformed to test biological
functions of PF4V1 and miR-875-3p in PCa. Western blotting was used to measure
downstream markers in AKT pathways and epithelial–mesenchymal transition (EMT).
In vivo experiments were performed to test the therapeutic effect of PF4V1
protein to PCa via a mouse model.
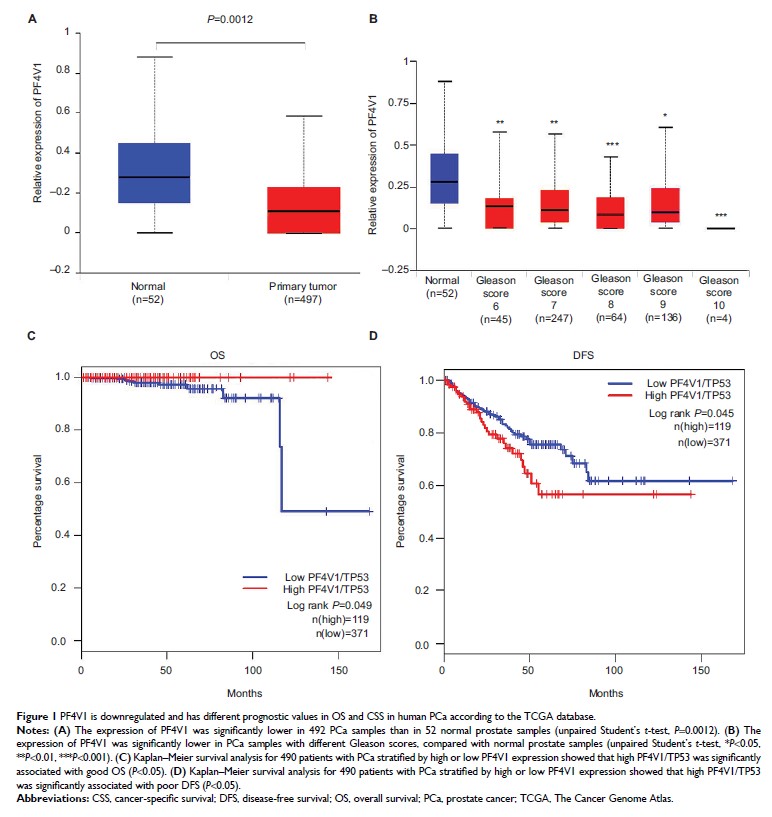
Results: The
expression of PF4V1 was significantly lower in 497 PCa samples than in 52
normal controls (P =0.0012). High PF4V1 expression (normalized by TP53)
was associated with poor disease-free survival (DFS) and good overall survival
(OS) in PCa (P <0.05).
PF4V1 was underexpressed in four PCa cell lines than in normal prostate cells.
Overexpression of PF4V1 could significantly suppress the proliferation,
migration, and invasion of DU145 and LNCaP cells (P <0.05).
Moreover, miR-875-3p targeted the 3’-untranslated region of PF4V1 and
derepressed the inhibitory function of PF4V1 in PCa (P <0.05). Key
proteins such as p-AKT/p-ERK/Snail/Slug/N-cadherin were downregulated, while
E-cadherin was upregulated when PF4V1 was overexpressed in PCa cells. Finally,
intratumoral injection of PF4V1 protein could significantly inhibit PCa growth
in vivo.
Conclusion: PF4V1 can
suppress the proliferation, migration, and invasion of PCa cells by regulating
AKT/ERK pathways and EMT. Elevated PF4V1/TP53 expression is correlated with
poorer DFS and better OS in the patients with PCa. The miR-875-3p-PF4V1 axis
may be a new therapeutic target site in PCa.
Keywords: PF4V1,
miR-875-3p, prostate cancer, survival