110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

LncRNA LOC105372579 通过激活 miR-4316/FOXP4 信号通路促进肝细胞癌的增殖和上皮 - 间质转化
Authors E C, Yang J, Li H, Li C
Received 12 December 2018
Accepted for publication 25 February 2019
Published 11 April 2019 Volume 2019:11 Pages 2871—2879
DOI https://doi.org/10.2147/CMAR.S197979
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 3
Editor who approved publication: Dr Ahmet Emre Eskazan
Background: Recently,
a growing number of long noncoding RNAs (lncRNAs) have been identified to be
important for human cancer development. However, how lncRNA regulates
hepatocellular carcinoma (HCC) progression still remains largely unclear. We
aimed to investigate the function of LOC105372579 in HCC progression.
Materials and methods: The
expression levels of lncRNA LOC105372579 in HCC tissues and cell lines were
analyzed by qRT-PCR. The effects of LOC105372579 silencing on proliferation,
migration and invasion were determined by using cell counting kit-8, colony
formation assay and Transwell assay. Moreover, the xenograft mouse model was
used to detect how LOC105372579 regulates HCC growth in vivo.
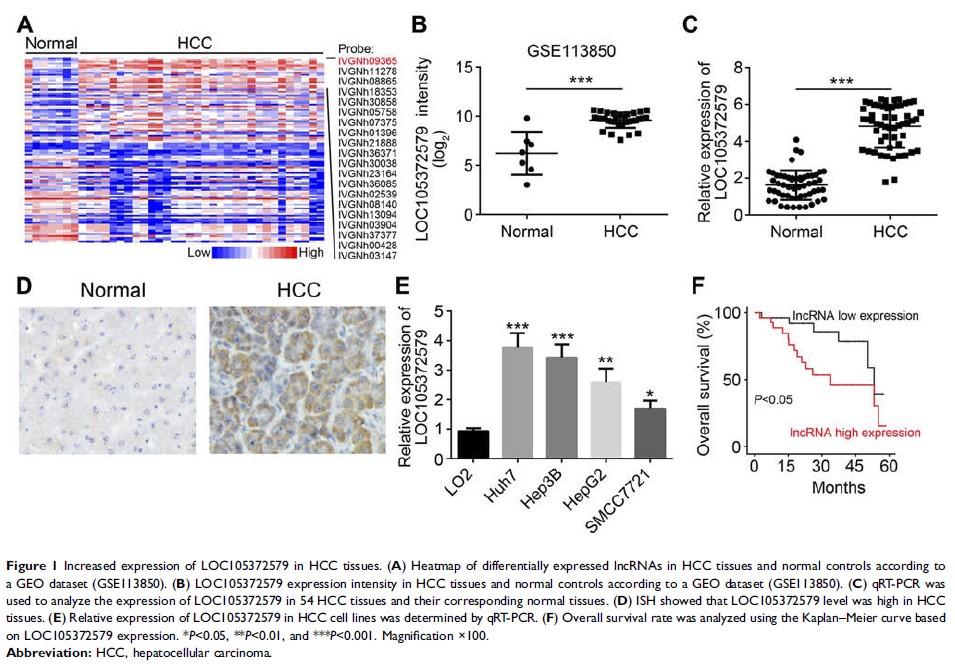
Results: LOC105372579
was highly expressed in HCC tissues and cell lines. Moreover, upregulated
levels of LOC105372579 predicted poor prognosis. LOC105372579 silencing
suppressed the proliferation of HCC cells in vitro. We also validated that
LOC105372579 knockdown inhibited the migration, invasion, and epithelial–mesenchymal
transition of HCC cells. Xenograft assay demonstrated that LOC105372579
promotes tumor growth in vivo. Mechanistically, we identified that LOC105372579
is a sponge for miR-4316 and that FOXP4 is a direct target of miR-4316.
Conclusion: Thus, our
findings supported that LOC105372579 contributes to HCC cell proliferation,
migration, invasion, and EMT by activating miR-4316/FOXP4 signaling.
Keywords: LOC105372579,
HCC, proliferation, EMT, progression