110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Pyrotinib 治疗 HER2 阳性胃癌细胞,促进释放的外泌体增强内皮细胞进展,这可以由阿帕替尼来抵消
Authors Gao Z, Song C, Li G, Lin H, Lian X, Zhang N, Cao B
Received 15 November 2018
Accepted for publication 12 March 2019
Published 11 April 2019 Volume 2019:12 Pages 2777—2787
DOI https://doi.org/10.2147/OTT.S194768
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Aims: Pyrotinib is a newly developed
irreversible pan-ErbB receptor tyrosine kinase inhibitor for treatment of human
epidermal growth factor receptor 2 (HER2)-positive cancers, and clinic trials
of pyrotinib in treatment of HER2-positive gastric cancer (GC) are underway.
Exosomes are tiny vesicles secreted by cancer cells and take essential roles in
the progression of carcinoma. Whether pyrotinib application has any effect on
the cancer cell-released exosomes has not been studied. The aim of our work was
to address if pyrotinib treatment impacts the effect of HER2-positive GC
cell-derived exosomes on endothelial cell (EC) progression.
Methods: Isolation
of exosomes released by HER2-positive NCI-N87 and MKN45 lines after pyrotinib
treatment was performed. Then, human umbilical vein endothelial cells (HUVECs)
were incubated with different concentrations of exosomes to address their
proliferation by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS). Effect of pyrotinib-treated exosomes at concentration of 10 μg/mL was
compared to that without pyrotinib treatment over 96-hr time course. Transwell
assay and wound-healing assay were carried out by incubating with exosomes
released by NCI-N87 and MKN45 cells with/without pyrotinib treatment over 24-hr
time course. The aforementioned experiments were done under same conditions in
order to evaluate the combined effect of apatinib and pyrotinib on HUVEC
motility and invasive capacity.
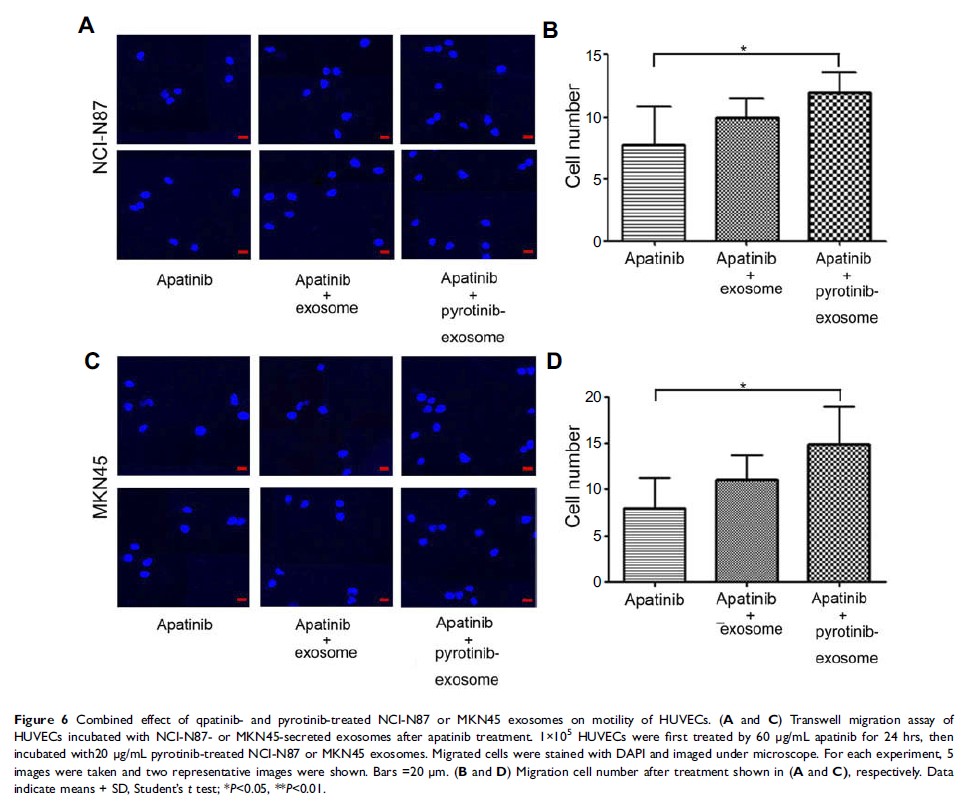
Results: We
showed that HUVEC proliferation, motility and invasive capacity were further
enhanced upon incubation with exosomes released by pyrotinib-treated GC cell
lines, compared to those without pyrotinib treatment. Significantly, this
effect was counteracted by the vascular endothelial growth factor receptor
(VEGFR)-2 inhibitor apatinib which inhibits EC progression.
Conclusion: Our
study suggests that pyrotinib application on HER2-positive GC produces stronger
exosomes that promote the proliferation and motility of vascular ECs, and
combination of pyrotinib with apatinib provides potentially better therapy.
Keywords: pyrotinib,
HER2, gastric cancer, GC, exosome, human umbilical vein endothelial cells,
HUVEC, apatinib