110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

顺铂,地塞米松,吉西他滨和培门冬酶(DDGP)在晚期(III-IV 期)结外 NK/T 细胞淋巴瘤初始治疗中的临床疗效及其与艾伯斯坦-巴尔病毒的相关性
Authors Zhao Q, Fan S, Chang Y, Liu X, Li W, Ma Q, Li Y, Wang Y, Zhang L, Zhang M
Received 24 October 2018
Accepted for publication 28 February 2019
Published 24 April 2019 Volume 2019:11 Pages 3555—3564
DOI https://doi.org/10.2147/CMAR.S191929
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 3
Editor who approved publication: Dr Beicheng Sun
Objective: To
evaluate the clinical efficacy and safety of the DDGP regimen in treating
extranodal NK/T-cell lymphoma and investigate the correlation between
Epstein-Barr virus (EBV)-DNA variation after treatment and the clinical
efficacy of NK/T-cell lymphoma.
Methods: Sixty-four
patients with extranodal NK/T-cell lymphoma received DDGP regimen-based
chemotherapy. Short-term and long-term clinical efficacy and adverse reactions
were observed. The relationship between EBV-DNA changes before and after
therapy and clinical efficacy was investigated.
Results: After the
DDGP regimen was used as the initial treatment, the short-term clinical
efficacy included 39 complete remission (CR) (60.94%), 12 partial remission
(PR) (18.75%), 2 stable disease (SD) (3.13%) and 11 progressive disease (PD)
(17.18%). Objective response rate (ORR) was 79.69% and 82.82% for disease
control rate (DCR). 3-year progression-free survival (PFS) was 62.00% and
3-year overall survive (OS) was 74.90%. Hemocytopenia was the predominant
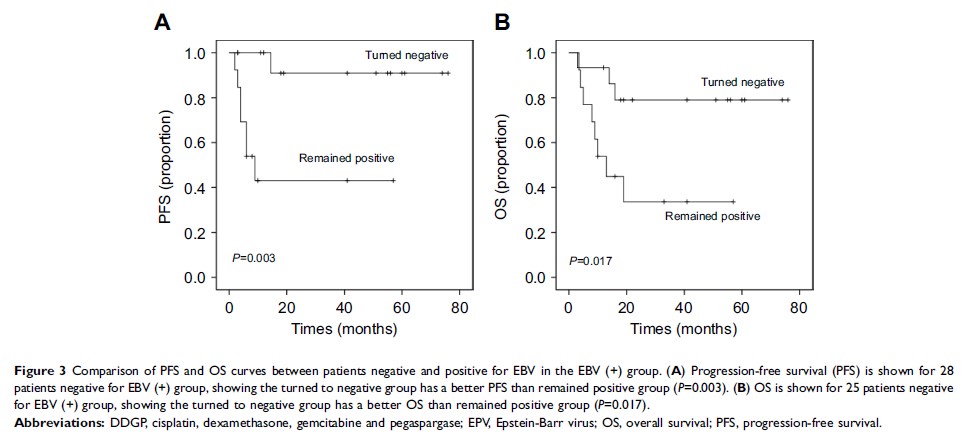
adverse effect. Between EBV-DNA positive group and its negative counterpart, a
significant difference was noted in OS (P =0.046), but no difference in ORR, DCR or PFS was
observed. In the EBV-DNA positive group, ORR, DCR, PFS and OS were higher for
patients whose EBV-DNA copy number decreased within a normal range than
patients remained positive (93.33% versus 61.53%, P =0.041 for ORR;
93.33% versus 61.53%, P =0.041 for DCR, P =0.003 for
PFS, P =0.017
for OS). The main adverse reactions included bone marrow suppression,
gastrointestinal reaction and coagulation dysfunction, which were mitigated and
treated after expectant or dose-decrement treatment.
Conclusion: DDGP
regimen can significantly improve the clinical prognosis of NK/T-cell lymphoma
patients with tolerable adverse reactions. The variation in EBV-DNA is
correlated with clinical efficacy and prognosis, which provides a theoretical
basis for NK/T-cell lymphoma therapy.
Clinical trial: In November
2011, this clinical trial was registered on the website: www.ClinicalTrials.gov
(No. NCT01501149).
Keywords: gemcitabine,
pegaspargase, extranodal NK/T-cell lymphoma, Epstein-Barr virus-DNA, prognosis