110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

伊马替尼和伏立康唑在体外和体内的药物相互作用研究
Authors Lin QM, Xie S, Qiu X, Chen J, Xu RA
Received 26 December 2018
Accepted for publication 27 March 2019
Published 30 April 2019 Volume 2019:12 Pages 1021—1027
DOI https://doi.org/10.2147/IDR.S199526
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Ms Justinn Cochran
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Background: In clinical
practice, common problem polypharmacy could result in the increased risks of
drug–drug interactions (DDIs). Co-administered imatinib (IMA) and voriconazole
(VOR) as one treatment protocol in cancer patients with fungal infections are
common.
Purpose: The aim of the
present study was to assess the potential DDIs associated with the concurrent
use of IMA and VOR in rat liver microsomes (RLMs) and in rats.
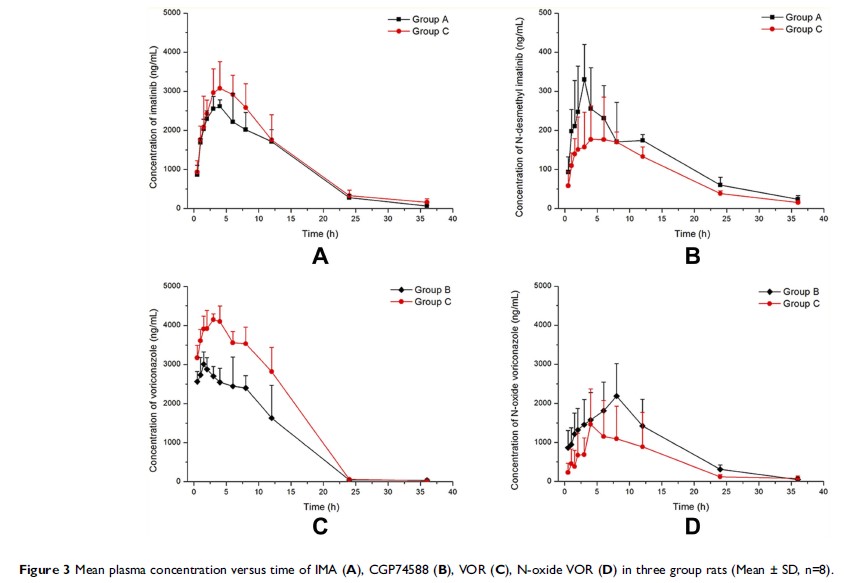
Methods and results: The
concentration levels of IMA, VOR, and their metabolites N-desmethyl IMA
(CGP74588) and N-oxide voriconazole (N-oxide VOR) were determined by ultra
performance liquid chromatography-tandem mass spectrometry. In vitro study of
RLMs, VOR inhibited the IMA metabolism with the half-maximal inhibitory
concentration (IC50) of 105.20 μM, while IC50 for IMA
against VOR was 61.30 μM. After co-administered IMA and VOR in rats, the C max of IMA was
increased significantly, while the AUC0→t, AUC0→∞, and C max of
CGP74588 were decreased significantly. In addition, similar results were also
found that the main pharmacokinetic parameters (AUC0→t, AUC0→∞, MRT0→∞, T max, and C max) of VOR were
increased significantly, while the AUC0→t, AUC0→∞, and C max of
N-oxide VOR were decreased significantly. Incorporation of all the results
indicated that both drugs had a inhibitory effect on each other’s metabolism in
vitro and in vivo.
Conclusion: Thus, it is of
great value to monitor the concomitant use of IMA and VOR in the clinic to
reduce the risks of unexpected clinical outcomes.
Keywords: drug–drug
interaction, imatinib, voriconazole, rat liver microsome, metabolism