110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

无药物甘露糖化脂质体通过促进肿瘤相关巨噬细胞的极化来抑制肿瘤生长
Authors Ye J, Yang Y, Dong W, Gao Y, Meng Y, Wang H, Li L, Jin J, Ji M, Xia X, Chen X, Jin Y, Liu Y
Received 4 March 2019
Accepted for publication 4 April 2019
Published 2 May 2019 Volume 2019:14 Pages 3203—3220
DOI https://doi.org/10.2147/IJN.S207589
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Alexander Kharlamov
Peer reviewer comments 2
Editor who approved publication: Dr Mian Wang
Background: Tumor-associated
macrophages (TAMs) are critical in tumor progression and metastasis. Selective
targeting of TAMs holds great potential to ameliorate the immunosuppressive
tumor microenvironment and enhance the efficacy of antitumor therapy. Various
liposomes have been developed to target TAMs via cell-specific surface
receptors either to deplete or re-educate TAMs. Since immuno-stimulation often
initiates with the interaction of nanocarriers with the innate immunity cells
such as macrophages, the intrinsic impact of drug-free liposomes on macrophage
activation and polarization via cell interaction is one of the most critical
issues in nanomedicine for promoting effective immunotherapy.
Methods: In this study,
conventional bare liposomes, PEGylated liposomes, and mannosylated liposomes
were developed and the cytotoxicity, cellular internalization,
immunostimulatory activity, targeting efficiency, antitumor efficacy, and
mechanism were evaluated in vitro and in vivo.
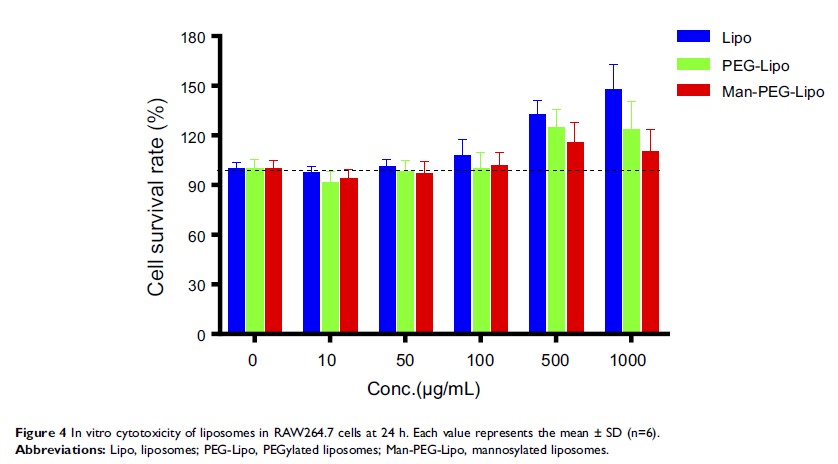
Results: All liposomes
displayed an ideal particle size, good biocompatibility, and controlled release
behavior. Mannosylated liposomes exhibited superior in vitro cellular
internalization and tumor spheroid penetration with the aid of the mannose
receptor-mediated TAMs-targeting effects. In particular, mannosylated liposomes
promoted the polarization of both M0 and M2 to the M1 phenotype by enhancing
the expression ratio of CD86/CD206 in vitro. Of note, mannosylated liposomes
could inhibit G422 glioma tumor growth, which may be attributed to the
polarization of TAMs, as evidenced by the reduction in expression level of the
TAMs surface marker.
Conclusion: These results
indicate the potential value of mannosylated liposomes in the design of a
rational delivery system to enhance the antitumor immune efficacy of
immunomodulators by inducing a shift from the M2 to the M1 phenotype.
Keywords: liposomes,
cancer immunotherapy, tumor-associated macrophages, mannose receptor, drug
delivery