110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

美托咪定降低七氟醚 EC50 的用量,用于病态肥胖患者自发呼吸的声门上气道装置插入
Authors Wan L, Shao LJZ, Liu Y, Wang HX, Xue FS, Tian M
Received 25 December 2018
Accepted for publication 20 March 2019
Published 3 May 2019 Volume 2019:15 Pages 627—635
DOI https://doi.org/10.2147/TCRM.S199440
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Professor Deyun Wang
Purpose: This
study aimed to assess the effect of intravenous dexmedetomidine (DEX) on
sevoflurane EC50 for supraglottic airway device (SAD)
insertion in spontaneously breathing morbidly obese patients.
Patients and methods: Thirty-eight
morbidly obese patients with a body mass index 40–57 kg/m2 who were
scheduled for bariatric surgery under general anesthesia requiring tracheal
intubation were randomly allocated to two groups receiving the different
treatments: group S, saline was given intravenously, and group D, a bolus dose
of DEX 1 μg/kg was administered intravenously over 10 mins, followed by
intravenous DEX infusion at a rate of 0.5 μg/kg/h. Five percent sevoflurane was
initially inhaled for anesthesia induction and then end-tidal expiratory sevoflurane
concentration (ETsev) was adjusted
to a target value as to the modified Dixon’s up-and-down method. Patients’
response to SAD insertion was classified as “movement” or “no movement”. The
average of the midpoints of all crossover points was defined as calculated
sevoflurane EC50 for successful SAD insertion.
Furthermore, the probit regression analysis was used to determine sevoflurane
end-tidal concentrations where 50% (EC50) and 95% (EC95) insertions of
SAD were successful. After the observation was completed, flexible
bronchoscope-guided intubation was performed through the SAD.
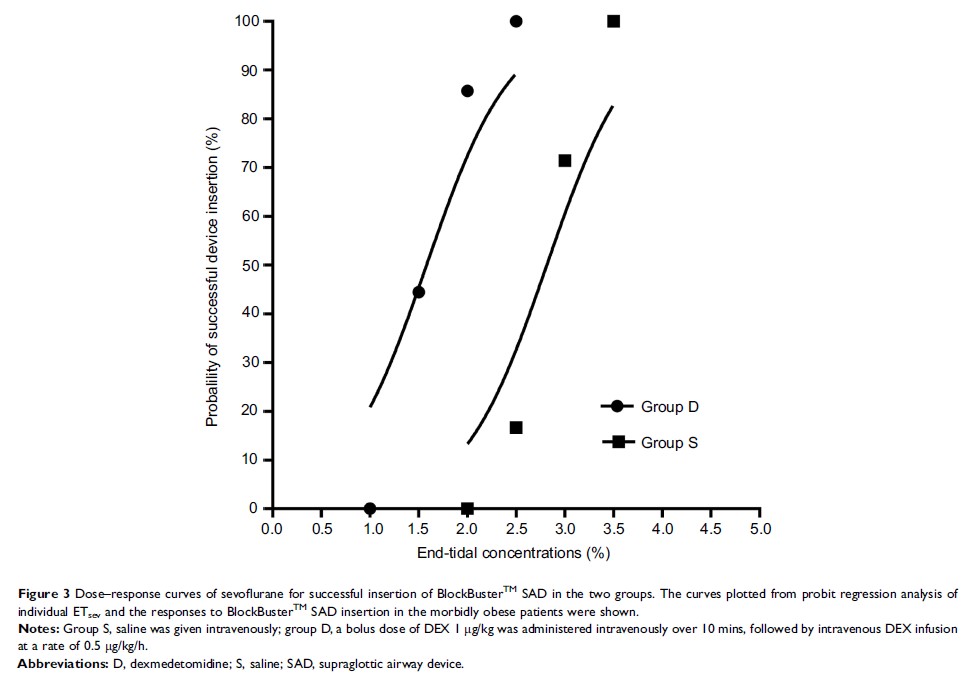
Results: The
calculated sevoflurane EC50 for successful SAD insertion was
significantly lower in group D than in group S (1.75±0.32% vs 2.92±0.26%, p <0.001). By the
probit regression analysis, EC50 and EC95 of
sevoflurane for successful SAD insertion were 1.59% (95% CI, 1.22–1.90%) and
2.15% (95% CI, 1.86–3.84%) in group D, respectively, and 2.81% (95% CI,
2.35–3.29%) and 3.32% (3.02–6.74%) in group S.
Conclusion: When
sevoflurane inhalational induction is performed in spontaneous breathing
morbidly obese patients, intravenous DEX can reduce sevoflurane EC50 for
successful SAD insertion by about 40%.
Chinese Clinical Trial Registry: No.
ChiCTR1800016868
Keywords: obesity,
inhalational induction, sevoflurane, dexmedetomidine, supraglottic airway
device