110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

程序性细胞死亡分子 1 抑制剂在晚期非小细胞肺癌患者中的疗效和安全性:荟萃分析
Authors Liu Y, Zhou S, Du Y, Sun L, Jiang H, Zhang B, Sun G, Wang R
Received 4 November 2018
Accepted for publication 11 April 2019
Published 21 May 2019 Volume 2019:11 Pages 4619—4630
DOI https://doi.org/10.2147/CMAR.S193394
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Andrew Yee
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Objective: This
study aims to perform systematic review and meta-analysis of all randomized
controlled trials that compare the efficacy and safety of programmed death 1
(PD-1) inhibitors versus chemotherapy alone in previously untreated advanced
non-small cell lung cancer (NSCLC).
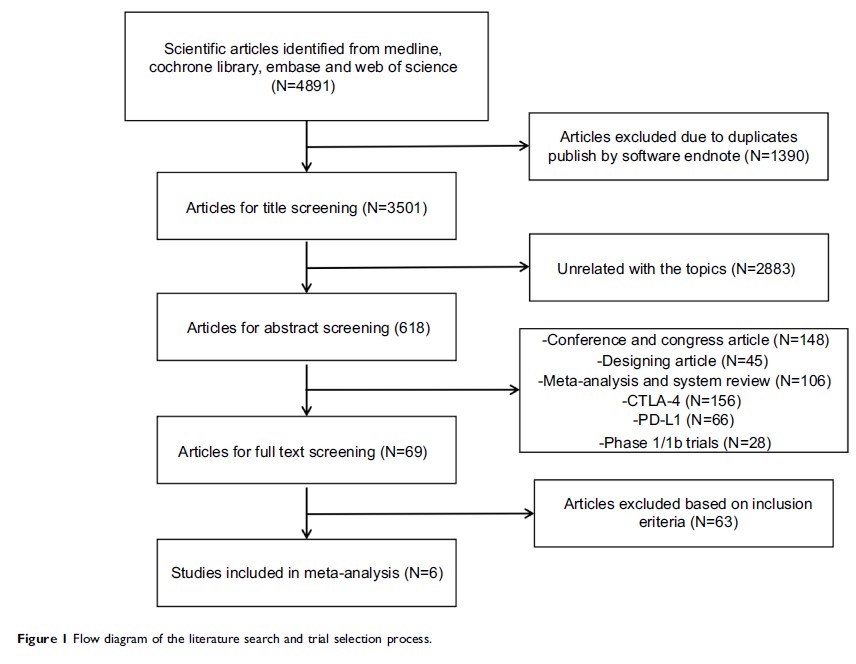
Materials and methods: Several
databases, including Medline, Cochrane Library, Embase, and Web of Science,
were searched. The main outcome measures included overall survival (OS),
progression-free survival (PFS), objective response rate (ORR), and adverse
events (AEs).
Results: The
results of meta-analysis are expressed as the hazard ratio (HR) or risk ratio
(RR) with their corresponding 95% confidence intervals (CIs). The final
analysis included six trials for 3,930 patients. PD-1 inhibitors led to a
statistically superior survival benefit over chemotherapy in patients with
advanced NSCLC. OS was longer in patients who received PD-1 inhibitors (HR
=0.71, 95% CI =0.62–0.74, P =0.000). Furthermore, PD-1 inhibitors had
significantly higher objective response rate than chemotherapy (RR =0.20,95% CI =0.17–0.23, P =0.000). Meta-analysis showed that the AEs of any
grade with PD-1 inhibitors were lower than those with chemotherapy (RR =0.78;
95% CI =0.75–0.81, P =0.000).
Conclusion: PD-1
inhibitors showed a clinically meaningful survival benefit and an improved
safety profile in patients with previously treated NSCLC.
Keywords: programmed
death 1, lung cancer, efficacy, safety