110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

白藜芦醇及其类似物在人膀胱癌细胞中的代谢特征及构效关系
Authors Yang Y, Zhang G, Li C, Wang S, Zhu M, Wang J, Yue H, Ma X, Zhen Y, Shu X
Received 26 February 2019
Accepted for publication 9 April 2019
Published 21 May 2019 Volume 2019:11 Pages 4631—4642
DOI https://doi.org/10.2147/CMAR.S206748
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Ms Justinn Cochran
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
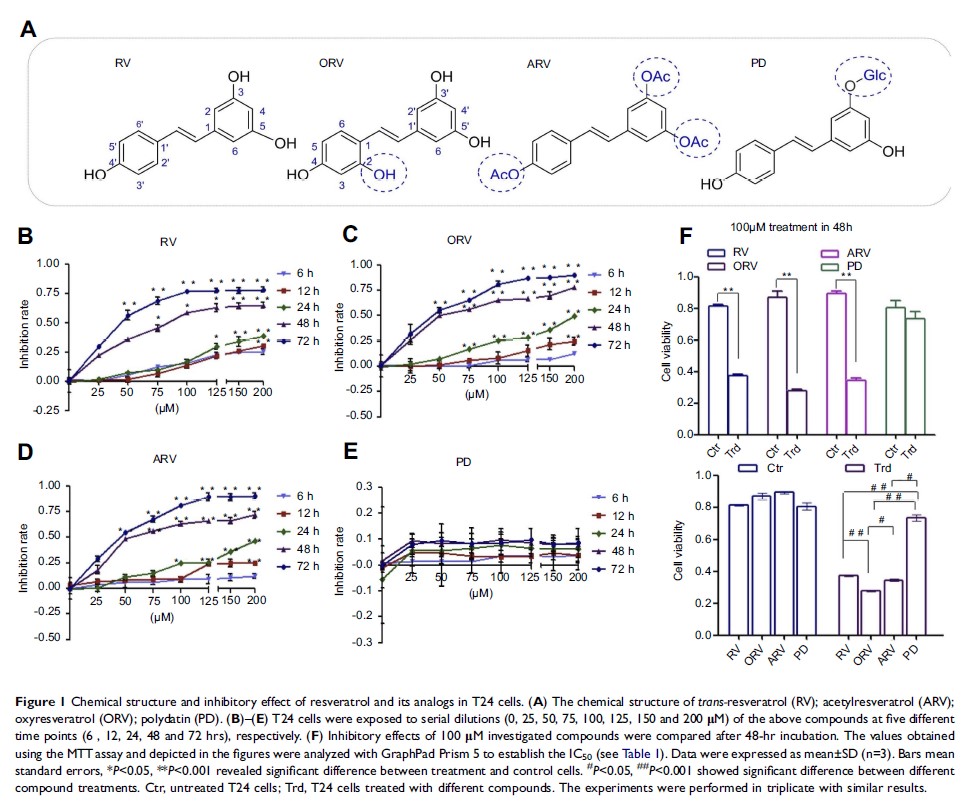
Purpose: Resveratrol
(RV), a promising anti-cancer candidate, is limited in application for its poor
bioavailability. However, the better bioavailability has been found in some RV
derivatives. So in this paper, we explore the structure–activity relationship
and the metabolic profiles of RV and its analogs (polydatin [PD],
oxyresveratrol [ORV], acetylresveratrol [ARV]) in human bladder cancer T24
cells, and then evaluate their active forms and key chemical functional groups
which may determine the fate of tumor cells.
Methods: Drug
sensitivity is evaluated by MTT assay, HE staining and flow cytometry analysis
after T24 cells treated with RV, PD, ORV and ARV, respectively. Then the drug
metabolites, in alive and dead T24 cells, also in T24 cell supernatant and
lysates, are qualitatively and quantitatively analyzed by high-performance
liquid chromatography, liquid chromatography coupled with tandem mass spectrum
and high-resolution mass spectrometry technologies, respectively.
Results: RV, ORV
and ARV inhibit bladder cancer cells growth in a dose- and time-dependent
manner, and exert the anti-tumor potency to T24 cells in order of
ORV>ARV>RV>PD. Meanwhile, similar metabolic profiles of the above
compounds are found not only in cell supernatant and lysate, but also in dead
and alive T24 cells after drug treatment, and the main metabolites of RV, ORV
and PD are their prototypes, but ARV is mainly metabolized to RV.
Conclusion: The
inhibitory potencies to T24 cells in the order of ORV>ARV>RV>PD are
related to the structure and metabolism of RV and its analogs. Meanwhile, the
number and position of free phenolic hydroxyl groups play a prominent role in
antitumor activities. Therefore, protecting phenolic hydroxyl groups, and
inhibiting drug metabolism to keep phenolic hydroxyl groups free would be the
promising strategies to ensure the bioactivity of RV and its analogs, and thus
to improve RV’s bioactivity and promote RV clinical translation.
Keywords: bladder
cancer, resveratrol, oxyresveratrol, acetylresveratrol, polydatin,
structure–activity relationship