110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

HEATR1 通过激活 p53/PUMA 信号传导途径调节非小细胞肺癌中的细胞存活
Authors He S, Ma X, Ye Y, Zhang M, Zhuang J, Song Y, Xia W
Received 23 November 2018
Accepted for publication 26 April 2019
Published 21 May 2019 Volume 2019:12 Pages 4001—4011
DOI https://doi.org/10.2147/OTT.S195826
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Amy Norman
Peer reviewer comments 2
Editor who approved publication: Dr William Cho
Aim: To
determine the mechanisms of HEATR1 on cell survival in non-small cell lung
cancer (NSCLC).
Methods: HEATR1
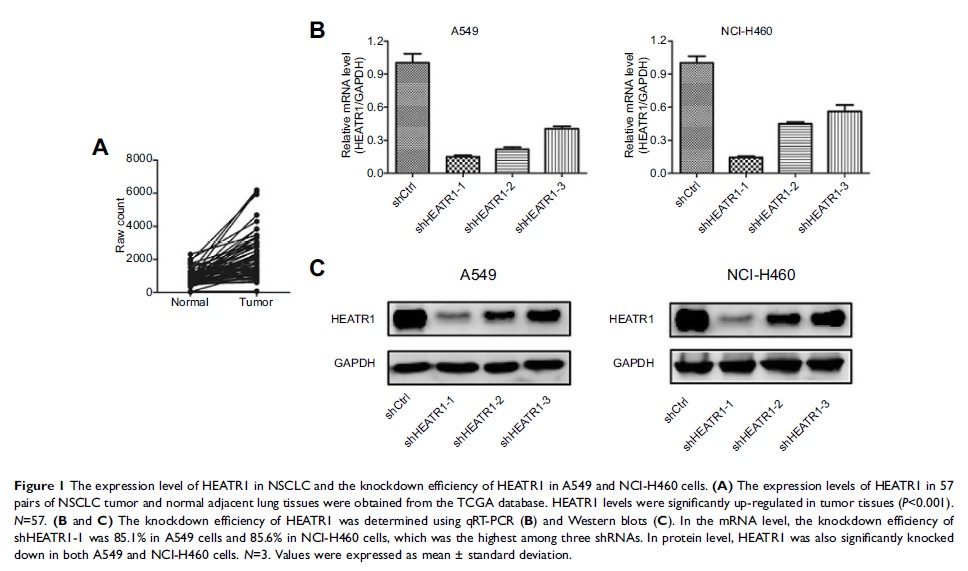
mRNA expression levels in 57 pairs of NSCLC tumor and adjacent normal lung
tissues were analyzed using the TCGA database. The effect of HEATR1 inhibition
on cell proliferation, apoptosis, and colony formation was measured in A549 and
NCI-H460 cells lines. In addition, the effect of HEATR1 inhibition on tumor
growth was measured using in vivo xenograft nude mouse models. Additionally,
downstream signaling pathways affected by HEATR1 inhibition were analyzed using
microarrays and bioinformatics analysis, and were validated using quantitative
real-time polymerase chain reaction and Western blot analysis.
Results: HEATR1
levels were significantly higher in NSCLC tumor tissues compared to normal
adjacent lung tissues (P <0.001). In vitro, cell proliferation was
significantly reduced in both A549 and NCI-H1299 cells transduced with shHEATR1
compared to shCtrl (P <0.001). Colony formation was also significantly
reduced after HEATR1 interference (P <0.01). Additionally, the percentage of apoptosis
was significantly increased in cells transduced with shHEATR1 (P <0.001). In
vivo, HEATR1 inhibition significantly reduced xenograft tumor growth in nude
mice. HEATR1 inhibition drastically affected the p53-signaling pathway,
significantly up-regulating PUMA and BAX both at the mRNA and protein levels (P <0.001), while
BCL2 levels were significantly down-regulated (P <0.01). The
cell proliferation and apoptosis were recovered in cell transduced with
shHEATR1 and shp53 compared to shHEATR1 (P <0.05).
Conclusion: HEATR1
inhibition activated p53 by reducing ribosome biogenesis, which subsequently
led to NSCLC cell apoptosis and reduced cell survival through the
p53-PUMA-BAX/BCL2 axis. Our results provide a mechanism by which therapeutic
modulation of HEATR1 could be a treatment strategy for NSCLC. In addition,
HEATR1 could be used as a potential biomarker for the prognosis or therapeutic
evaluation of NSCLC.
Keywords: HEATR1,
non-small cell lung cancer, p53, PUMA, cell survival