110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

加载地塞米松的可注射丝素 - 聚乙二醇水凝胶可缓解顺铂诱导的耳毒性
Authors Chen Y, Gu J, Liu J, Tong L, Shi F, Wang X, Wang X, Yu D, Wu H
Received 20 November 2018
Accepted for publication 21 April 2019
Published 6 June 2019 Volume 2019:14 Pages 4211—4227
DOI https://doi.org/10.2147/IJN.S195336
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 4
Editor who approved publication: Dr Linlin Sun
Background: Cisplatin is an extensively used anti-neoplastic agent for the treatment of various solid tumors. However, a high incidence of severe ototoxicity is accompanied by its use in the clinic. Currently, no drugs or therapeutic strategies have been approved for the treatment of cisplatin-induced ototoxicity by the FDA.
Purpose: The purpose of this study was to investigate the otoprotective effects of dexamethasone (DEX)-loaded silk-polyethylene hydrogel (DEX-SILK) following round window membrane administration in the cisplatin-induced ototoxicity mouse model.
Methods: The morphology, gelation kinetics, viscosity and secondary structure of the DEX-SILK hydrogel were analyzed. DEX concentration in the perilymph was tested at different time points following hydrogel injection on the RWM niche. Cultured cells (HEI-OC1), organ of Corti explants (C57/BL6, P0-2), and cisplatin-induced hearing loss mice model (C57/BL6) were used as in vitro and in vivo models for investigating the otoprotective effects of DEX-SILK hydrogel against cisplatin.
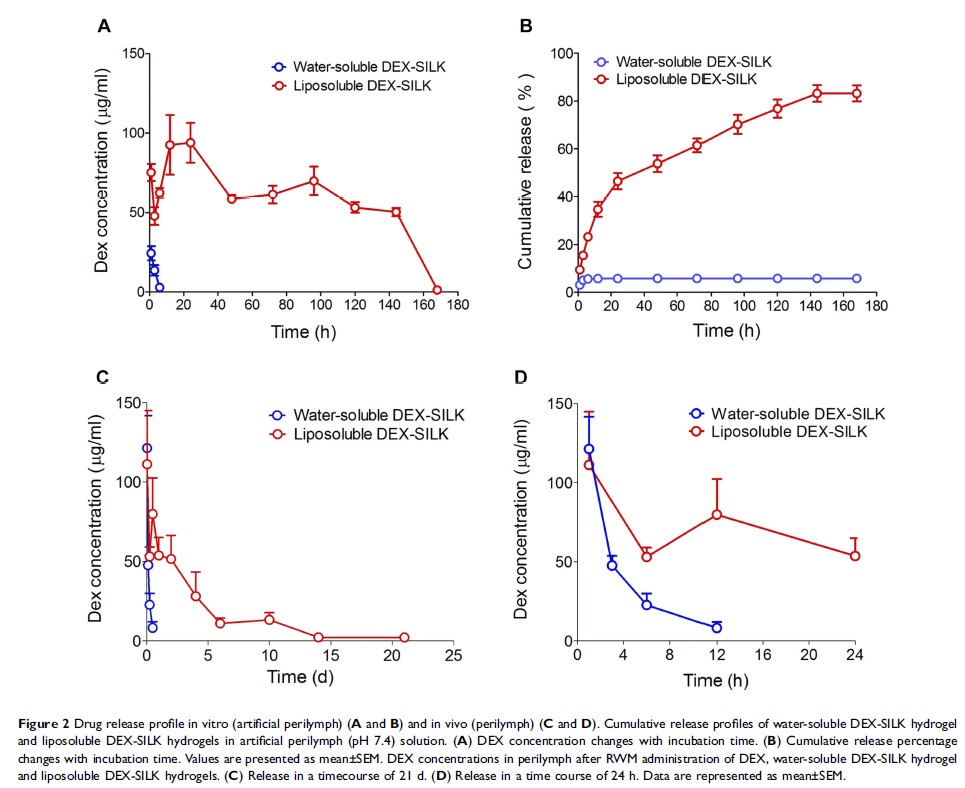
Results: Encapsulation of DEX with a loading of 8% (w/v) did not significantly change the silk gelation time, and DEX was evenly distributed in the Silk-PEG hydrogel as visualized by scanning electron microscopy (SEM). The concentration of Silk majorly influenced DEX distribution, morphological characteristics, viscosity, and gelation time. The optimized DEX-SILK hydrogel (8% w/v loading, 15% silk concentration, 10 μl) was administered directly onto the RWM of the guinea pigs. The DEX concentration in the perilymph was maintained above 1 μg/ml for at least 21 days for the DEX-SILK, while it was maintained for less than 6 h in the control sample of free DEX. DEX-SILK (5-60 ng/ml) exhibited significant protective effects against cisplatin-induced cellular ototoxicity and notably reduced the production of reactive oxygen species (ROS). Eventually, pretreatment with DEX-SILK effectively preserved outer hair cells in the cultured organ of Corti explants and demonstrated significant hearing protection at 4, 8, and 16 kHz in the cisplatin-induced hearing loss mice as compared to the effects noted following pretreatment with DEX.
Conclusion: These results demonstrated the clinical value of DEX-SILK for the therapy of cisplatin-induced ototoxicity.
Keywords: silk-polyethyleneglycol hydrogel, cisplatin-induced hearing loss, dexamethasone, round window membrane