110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

IGF1R 通过 STAT3/AKT 轴促进神经母细胞瘤的上皮 - 间质转化和癌症干细胞特性
Authors Wang XH, Wu HY, Gao J, Wang XH, Gao TH, Zhang SF
Received 3 December 2018
Accepted for publication 16 May 2019
Published 12 June 2019 Volume 2019:11 Pages 5459—5472
DOI https://doi.org/10.2147/CMAR.S196862
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 3
Editor who approved publication: Dr Chien-Feng Li
Background: Neuroblastoma (NB) displays the most heterogeneity in clinical manifestation. The insulin-like growth factor 1 receptor (IGF1R) has long been recognized for its role in tumourigenesis and growth. The IGF/IGF1R pathway is important in maintaining cell survival. It is reported that IGF1R participates in the occurrence of NB, but the mechanism is still unclear.
Methods: Human NB cell lines IMR-32 and SH-SY5Y were recruited in this study. IGF1R was knocked down by transfection with short hairpin RNA. Signal transducer and activator of transcription 3 (STAT3) expression was inhibited by Cryptotanshinone treatment. Cell proliferation, migration, and invasion were determined by MTT assay, wound healing assay, and cell invasion assay, respectively. The cancer stem cell properties were characterized by tumour sphere formation assay and colony formation assay. The mRNA and protein expression levels of related proteins were detected by RT-PCR and Western blot, respectively.
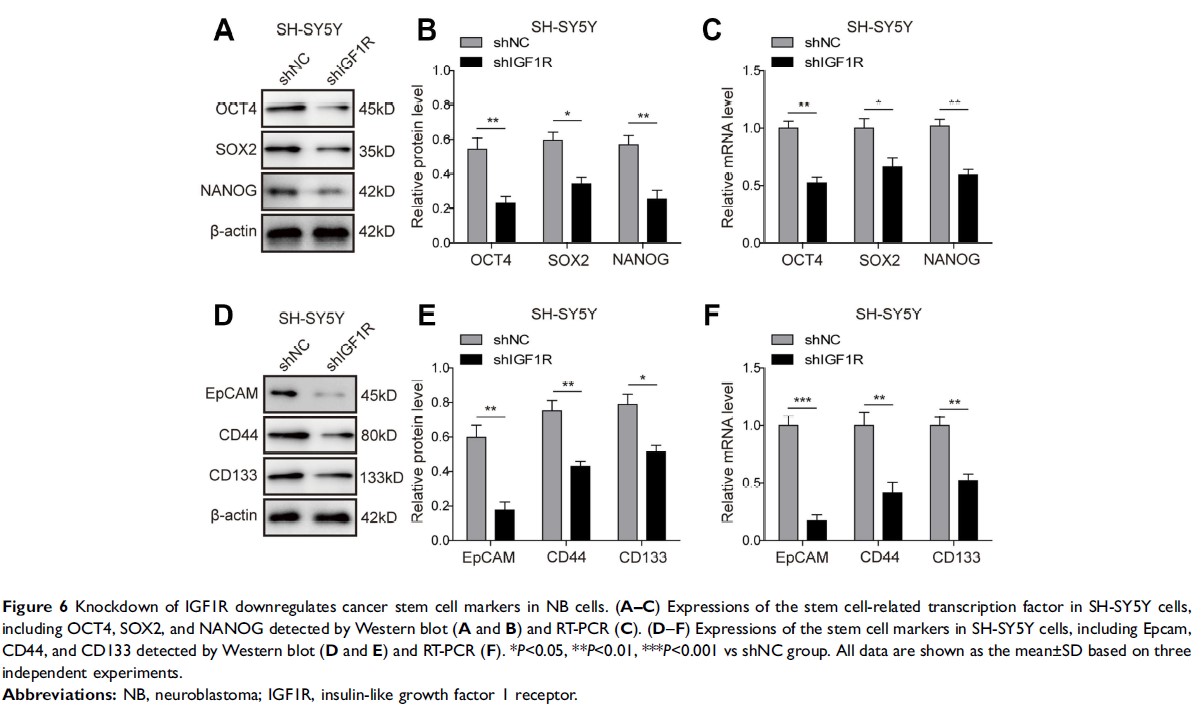
Results: The knockdown of IGF1R inhibits NB cell tumourigenesis and the epithelial-mesenchymal transition (EMT) of NB cells. Additionally, IGF1R was found to stimulate cancer stem cell-like properties in NPC cells. The knockdown of IGF1R significantly reduced the phosphorylation of AKT, and STAT3, indicating that the activation of the AKT and STAT3 pathways was inhibited by IGF1R knockdown. Furthermore, IGF1R was demonstrated to stimulate cancer stem cell-like properties in NB cells via the regulation of the STAT3/AKT axis.
Conclusion: IGF1R promotes cancer stem cell properties to facilitate EMT in neuroblastoma via the STAT3/AKT axis.
Keywords: IGF1R, neuroblastoma, epithelial mesenchymal transition, stemness, STAT3, AKT