110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

Evaluating guselkumab: an anti-IL-23 antibody for the treatment of plaque psoriasis
Authors Yang EJ, Smith MP, Ly K, Bhutani T
Received 2 December 2018
Accepted for publication 25 February 2019
Published 18 June 2019 Volume 2019:13 Pages 1993—2000
DOI https://doi.org/10.2147/DDDT.S137588
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Colin Mak
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Cristiana Tanase
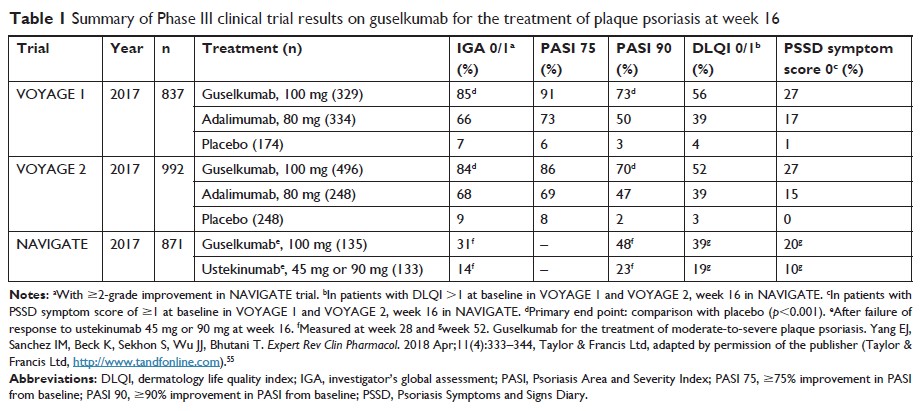
Abstract: The approval of guselkumab marks the entry of the IL-23 inhibitor class into the therapeutic armamentarium for patients with moderate-to-severe plaque psoriasis. This class specifically targets the upstream portion of the type 17 helper T (Th17) axis, which has been implicated as a key driver of the abnormal inflammatory state observed in psoriasis. Guselkumab is highly efficacious, with over 85% of the patients achieving ≥75% reduction in Psoriasis Area and Severity Index from baseline (PASI 75) and over 70% of the patients achieving PASI 90 response in its Phase III clinical trials. Additionally, this medication is well-tolerated, with non-serious infections such as nasopharyngitis and upper respiratory infections (URIs) being the most common adverse events (AEs) reported in its clinical trials. Guselkumab offers yet another effective treatment option in the rapidly growing list of available biological therapies for moderate-to-severe plaque psoriasis.
Keywords: biologics, guselkumab, IL-23 inhibitor, plaque psoriasis, biologic selection