111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

氨柔比星治疗既往未治疗的广泛性小细胞肺癌的安全性和有效性:荟萃分析
Authors Wu JF, Zhou JJ, Li XA, Hu LH, Wen ML
Received 6 January 2019
Accepted for publication 14 May 2019
Published 1 July 2019 Volume 2019:12 Pages 5135—5142
DOI https://doi.org/10.2147/OTT.S200601
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Giandomenico Roviello
Peer reviewer comments 2
Editor who approved publication: Dr Gaetano Romano
Background: Extensive-disease small-cell lung cancer (ED-SCLC) has been known to be rapid progression and relapse, despite highly sensitive to chemotherapy. Amrubicin (AMR), a third-generation synthetic anthracycline, was accepted as a feasible alternative compared with the standard first-line chemotherapy for previously untreated ED-SCLC. While, the efficacies of these amrubicin-based regimens are unsatisfactory.
Aim: Our meta-analysis was performed to assess the efficacy and toxicity of first-line therapy comparing AMR and chemotherapy in patients with ED-SCLC.
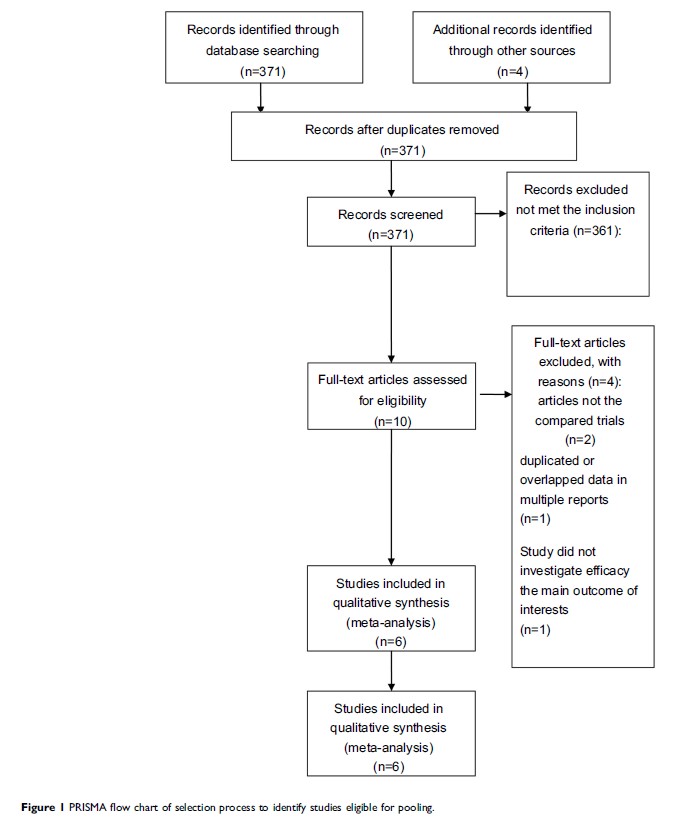
Methods: Electronic databases were searched for eligible trials updated on November 2018. Randomized-controlled trials assessing the efficacy and safety of AMR in ED-SCLC were included, of which the interested results were objective response rate (ORR), progression-free survival (PFS), overall survival (OS), and adverse events (AEs).
Results: A total of 6 randomized controlled trials were included in this analysis. There are no significant differences in OS (OR=1.03, 95% CI=0.66–1.60, P =0.91), PFS (OR=1.2, 95% CI=10.77–1.88, P =0.41) or ORR (OR=1.31, 95% CI=0.90–1.92, P =0.16) with AMR (OR=0.90, 95% CI=0.76–1.05, P =0.17). The most common treatment-related AEs in the AMR group are leukopenia (OR=3.13, 95% CI=1.22–7.99, P =0.02) and neutropenia (OR=3.25, 95% CI=1.38–7.65, P =0.007). Fatigue, anemia, nausea, vomiting, diarrhea the difference between the two groups had no statistical significance.
Conclusion: The results of our analysis indicated that AMR therapy demonstrated non-inferiority to the standard first-line chemotherapy for previously untreated ED-SCLC. Whether it can be accepted as an alternative regimen to the standard first-line chemotherapy is still warranted.
Keywords: small-cell lung cancer, extensive-disease, amrubicin, meta-analysis