110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

通过抑制 STAT3 通路设计、合成和评价氰基吡啶作为抗大肠癌的药物
Authors Xu L, Shi L, Qiu S, Chen S, Lin M, Xiang Y, Zhao C, Zhu J, Shen L, Zuo Z
Received 30 May 2019
Accepted for publication 3 September 2019
Published 24 September 2019 Volume 2019:13 Pages 3369—3381
DOI https://doi.org/10.2147/DDDT.S217800
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Nicola Ludin
Peer reviewer comments 2
Editor who approved publication: Professor Jianbo Sun
Background: Colorectal cancer is one of the common malignant tumors. Cyanopyridine and aminocyanopyridine having a carbon-nitrogen bond have been shown to have significant anticancer effects. STAT3 is a promising therapeutic target in multiple cancers. However, there are currently no effective STAT3 inhibitors in clinical practice for the treatment of colorectal cancer.
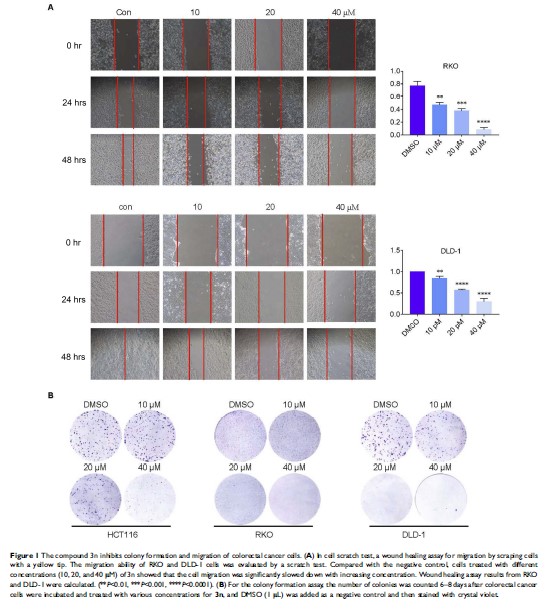
Materials and methods: We screened 27 cyanopyridines for their anticancer activity by cell viability. The HCT-116, RKO, and DLD-1 cell lines were used to evaluate the anti-colorectal cancer effect of 3n. Scratch experiments and colony formation assays were used for the assessment of cell migration and proliferation capacity. Phosphorylated STAT3, STAT3, MCL-1, and Survivin levels were assessed by Western blot analysis.
Results: In this study, we synthesized 27 cyanopyridines and screened their anticancer activities in three human tumor cells, HCT-116, Hela229, and A375. We found that 2-amino-3-cyanopyridine 3n has better anticancer activity with IC50 values in the low micromolar range. Furthermore, 3n significantly inhibited the migration and colony formation of colorectal cancer cells. Mechanistically, 3n inhibited the expression of STAT3 phosphorylation in a dose- and time-dependent manner.
Conclusion: 3n is worth of further investigations toward the discovery of STAT3 inhibitor as a drug candidate for cancer therapy.
Keywords: design, cyanopyridine, colorectal cancer, STAT3, inhibitor