110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

循环肿瘤细胞的计数、表征及其在晚期胃癌中的应用
Authors Cheng B, Tong G, Wu X, Cai W, Li Z, Tong Z, He L, Yu S, Wang S
Received 15 July 2019
Accepted for publication 13 September 2019
Published 25 September 2019 Volume 2019:12 Pages 7887—7896
DOI https://doi.org/10.2147/OTT.S223222
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Ms Aruna Narula
Peer reviewer comments 2
Editor who approved publication: Dr Takuya Aoki
Background: Advanced gastric cancer (aGC) has a high global incidence and a high mortality rate and because of its high malignancy and heterogeneity, the existing methods for prognosis are limited, and a new treatment model is necessary. Circulating tumor cells (CTCs) could be considered as a “liquid biopsy” for tumor diagnosis and for monitoring treatment responses and predicting clinical outcome. Clinical studies support the efficacy of programmed cell death 1 (PD-1) immunotherapy in a subset of aGC.
Methods: Cell capture efficiency, as described by the CanPatrol CTC enrichment technique, was validated using artificial blood samples as well as blood samples from 32 aGC patients. Clinicopathologic data of patients were collected from the hospital information system. We used CanPatrol for CTC isolation, classification, and clinical analysis.
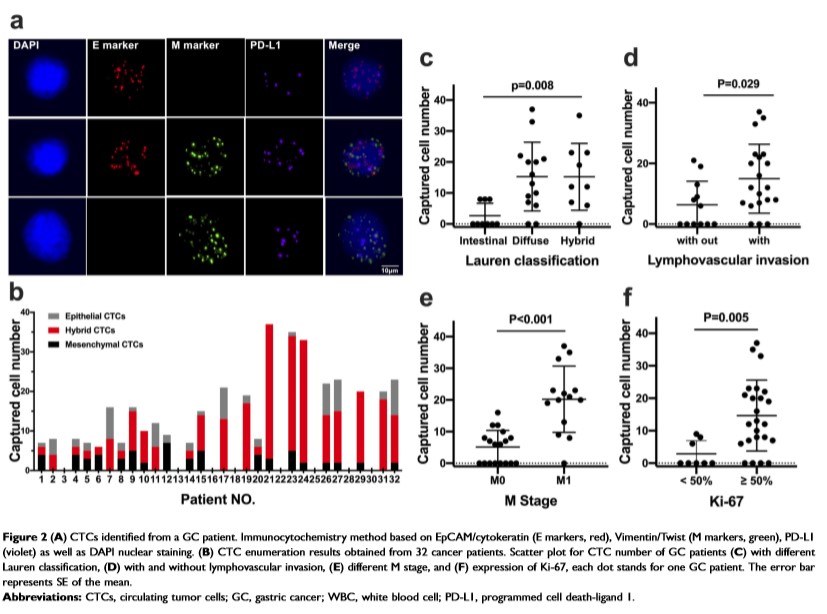
Results: A cell capture efficiency of >80% was achieved. Significant correlation was observed between CTC enumeration and clinicopathology parameters, including the Lauren classification (r=0.470, P=0.008), perineural invasion (r=0.393, P=0.029), TNM stage (r=0.740, P<0.001), and Ki-67 level (r=0.510, P=0.005). When compared to the traditional methods, monitoring CTC subtypes exhibits higher sensitivity of evaluating the disease status. Enumeration of epithelial CTC subset and its relative abundance in the total CTC pool are highly correlated with clinical efficacy. CTC programmed cell death ligand-1 (PD-L1) could be successfully detected for immunotherapy in addition to PD-L1 immunohistochemistry and microsatellite instability.
Conclusion: We provide a new method that allows for the simple and effective detection of CTCs in aGC. It has potential clinical applications in monitoring prognosis and guiding future individualized immunotherapy.
Keywords: CTC enumeration, clinical response prediction, subgroup analysis, immune checkpoint therapy