111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

通过靶向 FHL1,hsa-miR-664a-3p 的过表达与香烟烟雾诱导的慢性阻塞性肺病有关
Authors Zhong S, Chen C, Liu N, Yang L, Hu Z, Duan P, Shuai D, Zhang Q, Wang Y
Received 25 July 2019
Accepted for publication 12 September 2019
Published 9 October 2019 Volume 2019:14 Pages 2319—2329
DOI https://doi.org/10.2147/COPD.S224763
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Melinda Thomas
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Chunxue Bai
Background: Chronic obstructive pulmonary disease (COPD) is recognized as a chronic lung disease with incomplete reversible airflow limitation, but its pathophysiology was still not clear. This study aimed at investigating regulatory roles of special miRNA–mRNA axis in COPD development.
Methods: Differentially expressed miRNAs and downstream mRNAs were screened from the Gene Expression Omnibus (GEO) dataset by using the LIMMA package in R software. Weighted Gene Co-expression Network Analysis (WGCNA) was used to construct a co-expression network for COPD. The correlation of dysregulated miRNA(s) and COPD was analyzed, and miRNAs with significant differences were validated in peripheral blood mononuclear cells (PBMCs) from COPD patients by real-time PCR. Regulatory roles of candidate miRNAs and targeted mRNAs were investigated in vitro study.
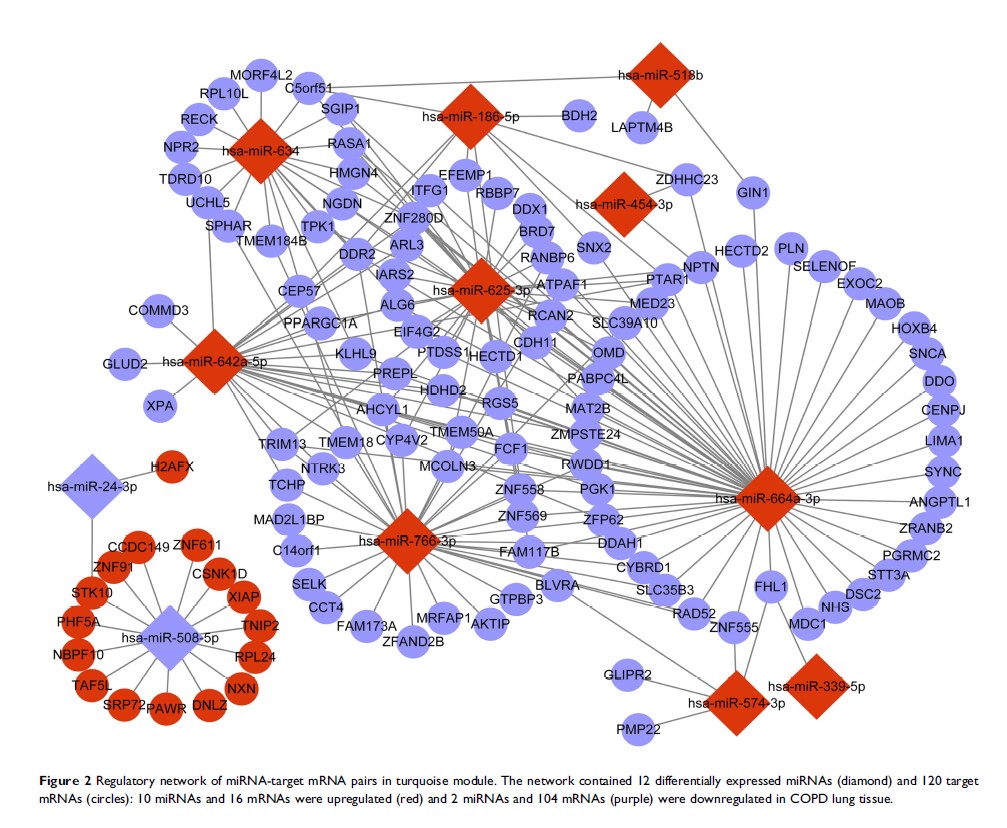
Results: Thirteen modules of co-expressed miRNAs and mRNAs were constructed from a selected cohort with WGCNA. Turquoise module with 12 differentially expressed miRNAs and 120 mRNAs was significantly correlated with COPD. The expression of hsa-miR-664a-3p, an upregulated miRNA in the module, was increased both in lung tissue and PBMCs from COPD patients, whereas that targeted four and a half LIM domains 1 (FHL1 ) gene was decreased and positively correlated with forced expiratory volume in 1 sec (FEV1)/forced vital capacity (FVC%) (r = 0.59, p < 0.01). In vitro, luciferase activity assay revealed FHL1 as a target of hsa-miR-664a-3p and it could be directly downregulated by overexpression of hsa-miR-664a-3p. Furthermore, cigarette smoke extract could increase hsa-miR-664a-3p level and decrease FHL1 level in Beas-2B cells.
Conclusion: The present study validated significant upregulation of hsa-miR-664a-3p in COPD patients, and its target gene FHL1 was downregulated and positively correlated with FEV1/FVC%; both hsa-miR-664a-3p and FHL1 could be regulated by cigarette smoke extract. Results of bioinformatic analyses and expanded validation suggest that the axis from hsa-miR-664a-3p to FHL1 might play a key role in cigarette smoke-induced COPD, and the exact mechanism should be confirmed in further studies.
Keywords: COPD, miRNA, mRNA, co-expression networks, biomarker