111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

多元素表达评分是多形性胶质母细胞瘤的一个预后因素
Authors Li JQ, Wang QT, Nie Y, Xiao YP, Lin T, Han RJ, Li Z, Fan YY, Yuan XH, Wang YM, Zhang J, He YW, Liao HX
Received 21 August 2019
Accepted for publication 9 October 2019
Published 17 October 2019 Volume 2019:11 Pages 8977—8989
DOI https://doi.org/10.2147/CMAR.S228174
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Purpose: Glioblastoma multiforme (GBM) is a highly malignant tumor of the central nervous system. Although primary GBM patients receive extensive therapies, tumors may recur within months, and there is no objective and scientific method to predict prognosis. Adoptive immunotherapy holds great promise for GBM treatment. However, the expression profiles of the tumor-associated antigens (TAAs) and tumor immune microenvironment (TME) genes used in immunotherapy of GBM patients have not been fully described. The present study aimed to develop a predictive tool to evaluate patient survival based on full analysis of the expression levels of TAAs and TME genes.
Methods: Expression profiles of a panel of 87 TAAs and 8 TME genes significantly correlated with poor prognosis were evaluated in 44 GBM patients and 10 normal brain tissues using quantitative real-time polymerase chain reaction (qRT-PCR). A linear formula (the LASSO algorithm based in the R package) weighted by regression coefficients was used to develop a multi-element expression score to predict prognosis; this formula was cross-validated by the leave-one-out method in different GBM cohorts.
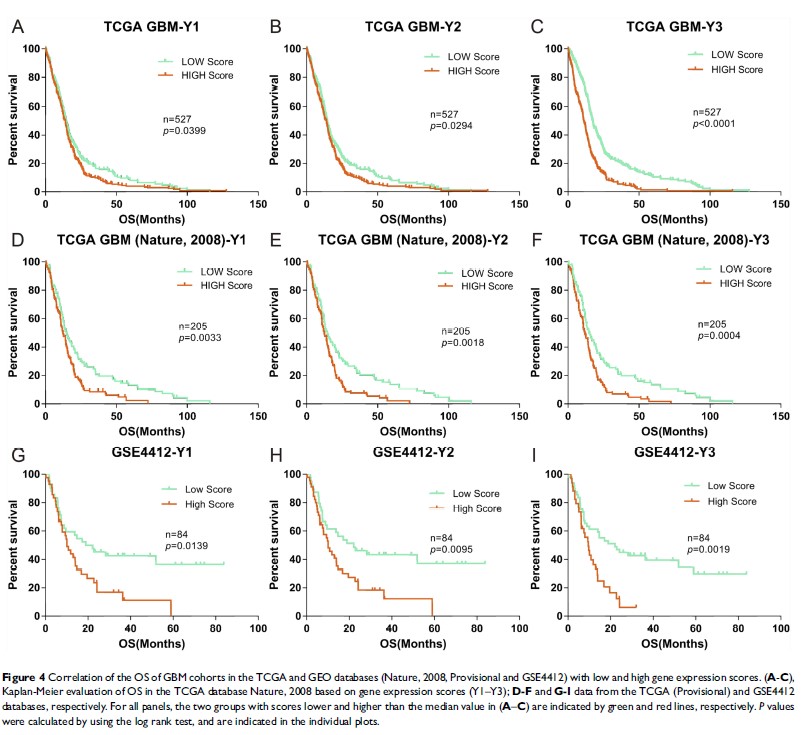
Results: After analysis of gene expression, clinical features, and overall survival (OS), a total of 8 TAAs (CHI3L1, EZH2, TRIOBP, PCNA, PIK3R1, PRKDC, SART3 and EPCAM), 1 TME gene (FOXP3) and 4 clinical features (neutrophil-to-lymphocyte (NLR), number of basophils (BAS), age and treatment with standard radiotherapy and chemotherapy) were included in the formula. There were significant differences between high and low scoring groups identified using the formula in different GBM cohorts (TCGA (n=732) and GEO databases (n=84)), implying poor and good prognosis, respectively.
Conclusion: The multi-element expression score was significantly associated with OS of GBM patients. The improve understanding of TAAs and TMEs and well-defined formula could be implemented in immunotherapy for GBM to provide better care.
Keywords: glioblastoma, gene expression score, prognosis, TAAs, TME