111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

负载葛根素并由三苯基磷阳离子修饰的胶束具有线粒体靶向功能,并显示出对异戊二烯诱导的 H9c2 细胞凋亡的增强保护作用
Authors Li W, Wu J, Xiang D, Luo S, Hu X, Tang T, Sun T, Liu X
Received 16 June 2019
Accepted for publication 16 September 2019
Published 21 October 2019 Volume 2019:14 Pages 8345—8360
DOI https://doi.org/10.2147/IJN.S219670
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Alexander Kharlamov
Peer reviewer comments 2
Editor who approved publication: Dr Lei Yang
Background: The protective role of puerarin (PUE) against myocardial infarction is closely related to its regulation on mitochondria. However, free PUE can hardly reach the mitochondria of ischemic cardiomyocytes due to the lack of mitochondrial targeting of PUE. Here PUE was loaded into mitochondria-targeted micelles (PUE@TPP/PEG-PE) for precisely delivering PUE into mitochondria with the aim of enhancing the anti-apoptosis effect.
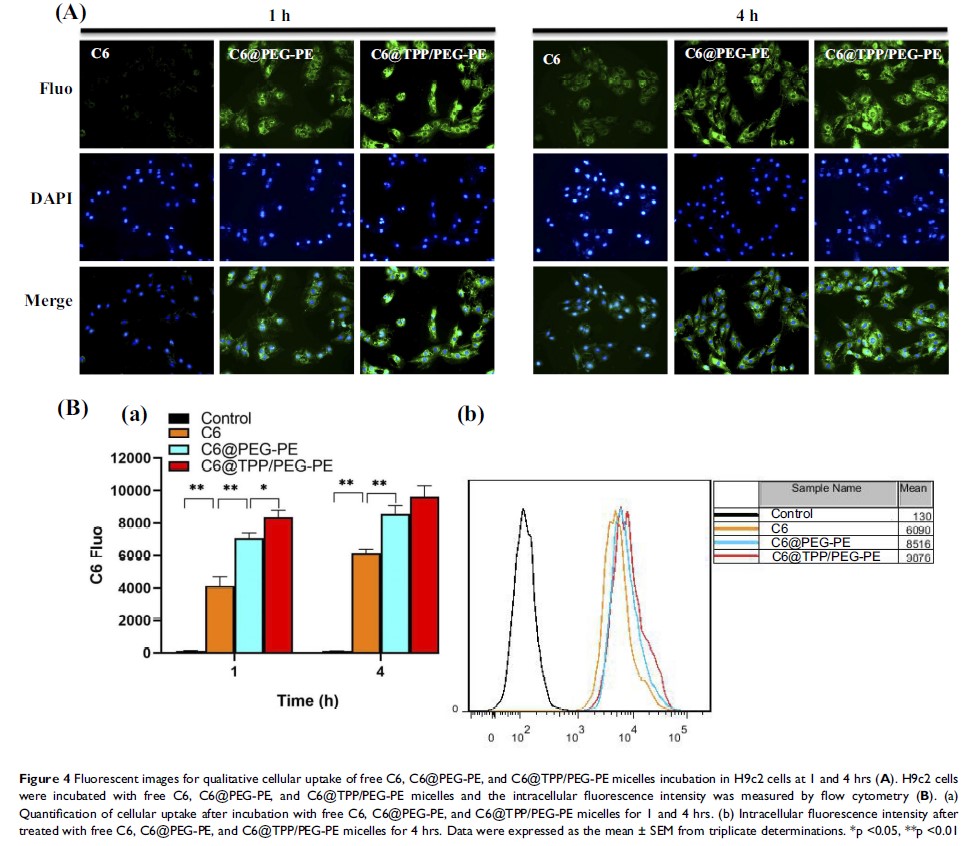
Methods: The mitochondriotropic polymer TPP-PEG-PE was synthesized for the preparation of PUE@TPP/PEG-PE micelles modified with triphenylphosphonium (TPP) cation. The physicochemical properties and anti-apoptosis effect of PUE@TPP/PEG-PE micelles were investigated. The coumarin 6 (C6)-labeled TPP/PEG-PE (C6@TPP/PEG-PE) micelles were used to observe the enhanced cellular uptake, mitochondrial targeting and lysosomes escape. Moreover, in vivo and ex vivo biodistribution of lipophilic near-infrared dye 1,1ʹ-dioctadecyl-3,3,3′,3ʹ-tetramethylindotricarbocyanine iodide (DiR)-labeled PUE@TPP/PEG-PE (DiR@TPP/PEG-PE) micelles were detected through fluorescence imaging.
Results: The successful synthesis of TPP-PEG-PE conjugate was confirmed. PUE@TPP/PEG-PE micelles had a particle size of 17.1 nm, a zeta potential of −6.2 mV, and a sustained-release behavior. The in vitro results showed that the intracellular uptake of C6@TPP/PEG-PE micelles was significantly enhanced in H9c2 cells. C6@TPP/PEG-PE micelles could deliver C6 to mitochondria and reduce the capture of lysosomes. In addition, compared with the PUE@PEG-PE micelles and free PUE, the PUE@TPP/PEG-PE micelles exerted an enhanced protective effect against isoprenaline-induced H9c2 cell apoptosis, as evident by the decreased percentage of apoptotic cells, Caspase-3 activity, ROS level, Bax expression, and increased Bcl-2 expression. The in vivo detecting results of the targeting effect using DiR probe also indicated that TPP/PEG-PE micelles could accumulate and retain in the ischemic myocardium.
Conclusion: The results of this study demonstrate the promising potential of applying PUE@TPP/PEG-PE micelles in mitochondria-targeted drug delivery to achieve maximum therapeutic effects of PUE.
Keywords: mitochondrial targeting, anti-apoptosis, ischemic myocardium, TPP