111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

及早开始替诺福韦治疗可对 HBV 病毒载量高的孕妇带来更好的病毒抑制效果:一项真实世界的前瞻性研究
Authors Gao F, Zhang WT, Lin YY, Wang WM, Xu N, Bai GQ
Received 3 September 2019
Accepted for publication 25 October 2019
Published 7 November 2019 Volume 2019:12 Pages 3475—3484
DOI https://doi.org/10.2147/IDR.S228982
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Melinda Thomas
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Purpose: To investigate whether tenofovir disoproxil fumarate (TDF) treatment that started from the second trimester had an advantage over TDF treatment that started from the third trimester.
Patients and methods: Twenty 35-year-old pregnant women with hepatitis B virus (HBV) DNA >2×106 IU/mL were prospectively enrolled in this study. All participants were divided into two subgroups: the second trimester group who started TDF treatment at 24–27 weeks and the third trimester group who started TDF treatment at 28–30 weeks. The primary outcome was the change in serum HBV DNA level from baseline to delivery. Each parameter was tested every 4 weeks from TDF initiation to 3 months postpartum.
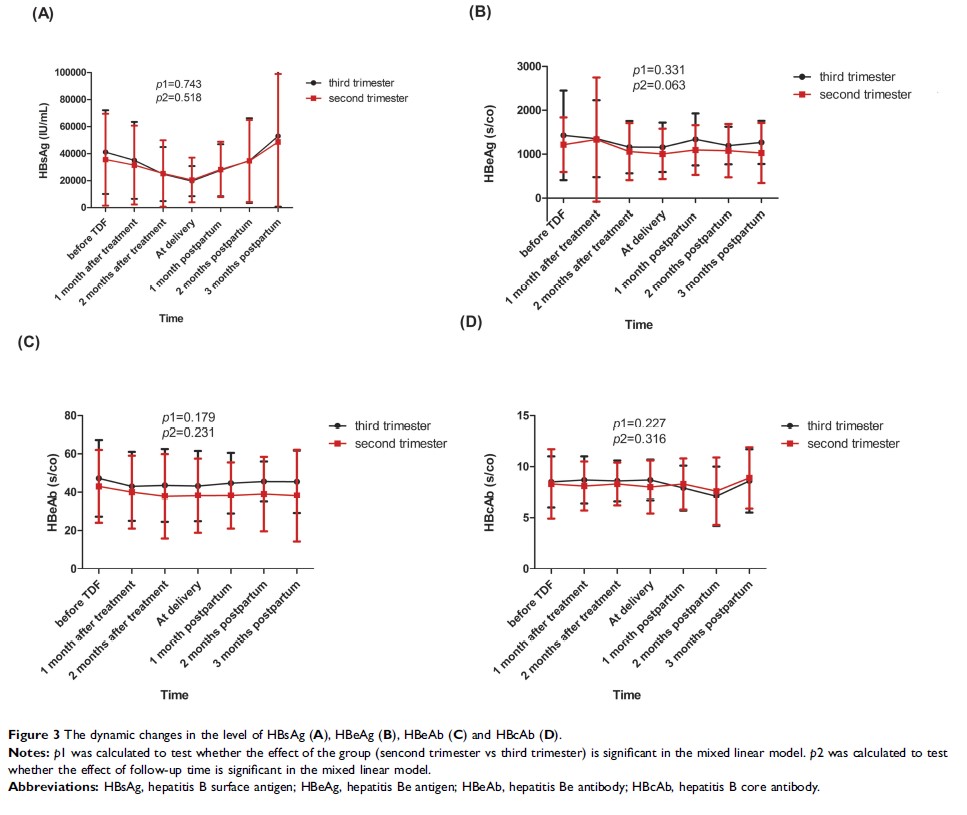
Results: There were 80 pregnant women in the second trimester group and 49 pregnant women in the third trimester group. The decline in HBV DNA from baseline to delivery was more obvious in the second trimester group (4.8±1.2 log10 IU/mL) than that in the third trimester group (4.3±1.1 log10 IU/mL, p=0.041). The downward shift of haemoglobin (HB) from baseline to delivery was greater in the second trimester group (10.6±10.7 g/L) than in the third trimester group (6.3±12.3 g/L, p=0.041). The decline in HBV DNA from baseline to delivery was linearly related to the start of TDF treatment from the second trimester (β=0.50 and 95% CI: 0.26–0.75, p<0.001). There were no significant differences between the two groups regarding HBV serologic markers and safety indicators.
Conclusion: Starting TDF treatment from the second trimester achieved better viral suppression than starting TDF treatment from the third trimester in highly viraemic pregnant women without increasing additional adverse reactions. HB level needed frequent monitoring during treatment to avoid anaemia.
Registry number: Clinical Trial No. NCT02719808.
Keywords: tenofovir disoproxil fumarate, efficacy, safety, second trimester, third trimester