111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

CCDC132 的敲除通过促进 DNA 损伤信号传导来减弱胃癌细胞的增殖和肿瘤发生
Authors Xu X, Cao W, Sun W, Wang Z, Chen H, Zheng Z, Yang X
Received 14 May 2019
Accepted for publication 15 October 2019
Published 12 November 2019 Volume 2019:11 Pages 9585—9597
DOI https://doi.org/10.2147/CMAR.S215631
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Melinda Thomas
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Background: Aberrant endocytic recycling has fundamental functions on plasma membrane component turnover. Recent studies have identified an uncharacterized protein, CCDC132, in the endosome-associated recycling protein complex. Besides, our preliminary data first showed that CCDC132 was elevated in malignant neoplasms, especially in esophagus/stomach cancers. However, the functions and the underlying mechanisms of CCDC132 in gastric cancer (GC) biology remain unclear.
Methods: The CCDC132 mRNA expression in 4 GC cell lines and normal gastric epithelial cell lines was detected by qRT-PCR. Then, CCDC132 was downregulated in AGS and MGC-803 cells by lentivirus-induced RNA interfere, and cell viability assay, clone formation assay and apoptosis assay were carried out. The mechanism of CCDC132 on cell proliferation and apoptosis activation was explored using PathScan® Stress, apoptosis signaling arrays and Western blot. We further investigated the pro-oncogenesis of CCDC132 in vivo. Meanwhile, immunohistochemistry was utilized to analyze the association between CCDC132 expression and clinicopathological features and prognosis. Finally, the correlation between CCDC132 and p53 was analyzed by Spearman’s rank correlation analysis.
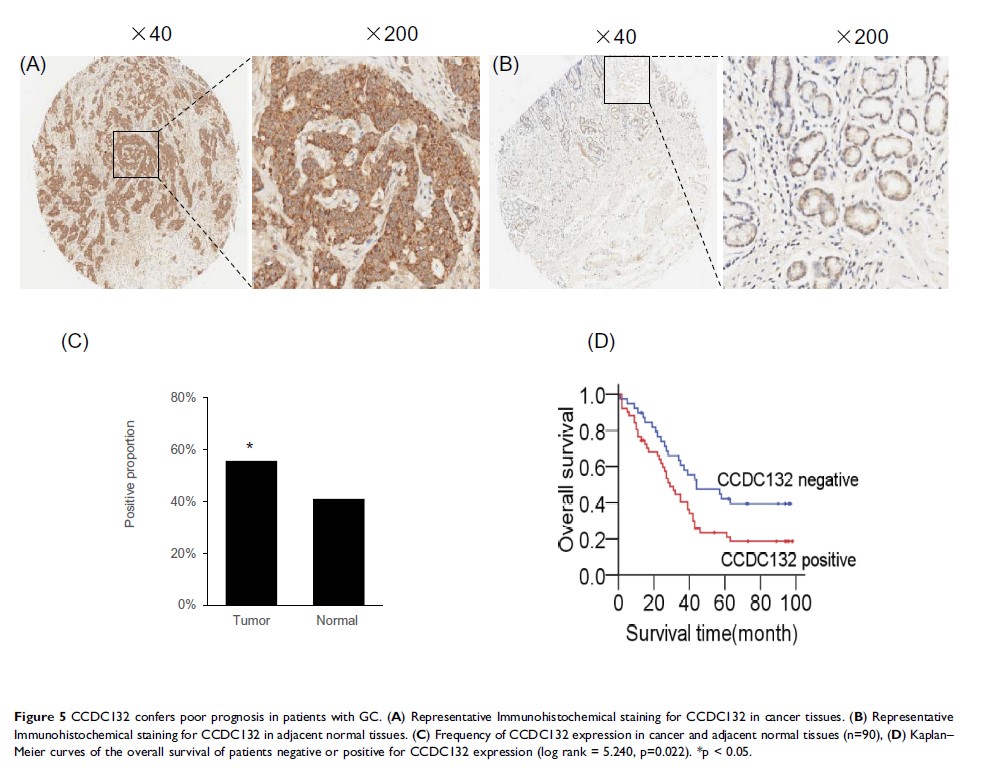
Results: In this study, knockdown of CCDC132 significantly decreased cell proliferation and clone formation ability and facilitated apoptosis, and increased phosphorylation of p53 and Chk2 and protein levels of γ-H2AX, 53BP1, cleaved Caspase 3 and cleaved PARP. Additionally, knockdown of CCDC132 attenuated tumorigenesis and tumor growth of MGC-803 cell xenografts. CCDC132 expression was significantly higher in GC tissues compared with that in adjacent normal tissues and was positively correlated with nodal metastasis and TNM stage and negatively associated with prognosis. The survival rate of CCDC132 positive patients was lower than that of CCDC132-negative patients. Furthermore, CCDC132 expression was negatively related to p53.
Conclusion: This study unravels that knockdown of CCDC132 attenuates GC cell proliferation and tumorigenesis by facilitating DNA damage signaling, indicating that CCDC132 may serve as a potential target for GC therapy.
Keywords: CCDC132, gastric cancer, proliferation, DNA damage, tumorigenesis