111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

过度表达的 miR-122-5p 通过负调控 PKM2 促进肾癌的细胞活力、增殖、迁移和糖酵解
Authors Wang S, Zheng W, Ji A, Zhang D, Zhou M
Received 2 August 2019
Accepted for publication 25 October 2019
Published 15 November 2019 Volume 2019:11 Pages 9701—9713
DOI https://doi.org/10.2147/CMAR.S225742
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Objective: Renal cancer is one of the most deadly urological malignancies. Currently, there is still a lack of effective treatment. Our purpose was to explore the mechanisms of miR-122-5p in renal cancer.
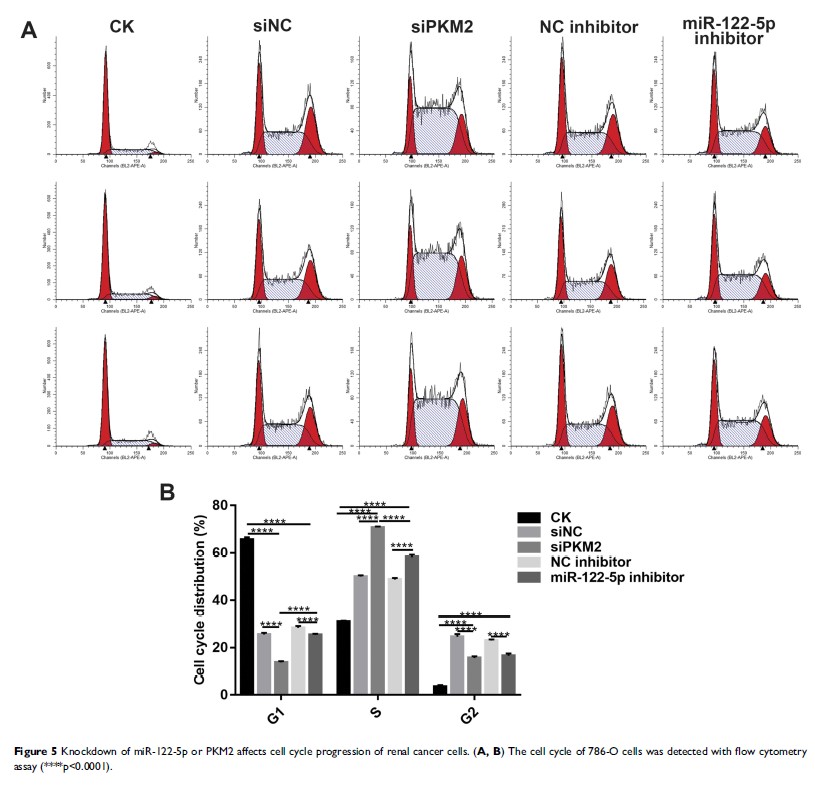
Methods: The expression levels of miR-122-5p and pyruvate kinase M2 (PKM2) in renal cancer cells were detected by RT-qPCR and Western blot analyses, respectively. Then, we measured the cell viability after knockdown of miR-122-5p and PKM2 using CCK-8 assay. Moreover, flow cytometry was used to investigate cell cycle and apoptosis of renal cancer cells. The cell migration of renal cancer cells transfected by miR-122-5p inhibitor and siPKM2 was then detected by wound healing assay. Furthermore, glucose consumption and lactate production were measured. Autophagy-related protein LCII/I was detected by Western blot.
Results: MiR-122-5p was upregulated in renal cancer cells compared to HK2 cells, especially in 786-O cells. We found that silencing miR-122-5p promoted PKM2 expression in 786-O cells. After transfection of siPKM2 or miR-122-5p inhibitor, the cell viability of 786-O cells was significantly reduced. Furthermore, the G1 phase of 786-O cells was significantly blocked, and the S phase was significantly increased. In addition, knockdown of miR-122-5p or PKM2 promoted renal cancer cell apoptosis and inhibited cell migration. Glucose consumption of 786-O cells was significantly increased after transfection by siPKM2. Silencing miR-122-5p significantly promoted the expression levels of LCII/I.
Conclusion: Our findings revealed that overexpressed miR-122-5p promotes renal cancer cell viability, proliferation, migration, glycolysis and autophagy by negatively regulating PKM2, which provide a new insight for the development of renal cancer therapy.
Keywords: PKM2, miR-122-5p, cell viability, glycolysis, renal cancer