111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

负载聚乙二醇(PEG)化 PEI 的超声微泡作为高效的基因传递系统
Authors Liufu C, Li Y, Tu J, Zhang H, Yu J, Wang Y, Huang P, Chen Z
Received 27 May 2019
Accepted for publication 2 September 2019
Published 15 November 2019 Volume 2019:14 Pages 8923—8941
DOI https://doi.org/10.2147/IJN.S217338
Checked for plagiarism Yes
Review by Single-blind
Peer reviewers approved by Dr Cristina Weinberg
Peer reviewer comments 2
Editor who approved publication: Dr Mian Wang
Background: Cancer stem cells (CSCs) are responsible for cancer therapeutic resistance and metastasis. To date, in addition to surgery, chemotherapy, and radiotherapy, gene delivery has emerged as a potential therapeutic modality for ovarian cancer. Efficient and safe targeted gene delivery is complicated due to the tumor heterogeneity barrier. Ultrasound (US)-stimulated microbubbles (MBs) have demonstrated a method of enabling non-invasive targeted gene delivery.
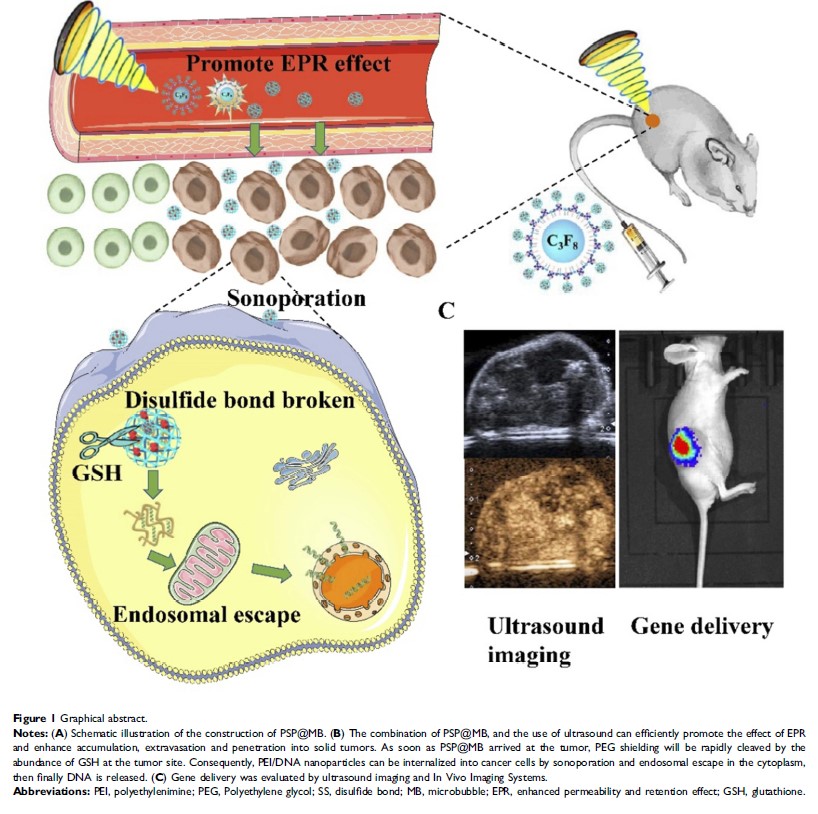
Purpose: The purpose of our study was to show the utility of poly(ethylene glycol)-SS-polyethylenimine-loaded microbubbles (PSP@MB) as an ultrasound theranostic and redox-responsive agent in a gene delivery system.
Patients and methods: PSP nanoparticles were conjugated to the MB surface through biotin–avidin linkage, increasing the gene-loading efficiency of MB. The significant increase in the release of genes from the PSP@MB complexes was achieved upon ultrasound exposure. The positive surface charge in PSP@MB can condense the plasmid through electrostatic interactions; agarose-gel electrophoresis further confirmed the ability of PSP@MB to condense plasmids. The morphology, particle sizes and zeta potential of PSP@MB were characterized by transmission electron microscopy and dynamic light scattering.
Results: Laser confocal microscopy showed that the combination of ultrasound with PSP@MB could promote the cellular uptake of plasmids. Plasmids which encode enhanced green fluorescence protein (EGFP) reporter genes or luciferase reporter genes were delivered to CSCs in vitro and to subcutaneous xenografts in vivo via the combination of ultrasound with PSP@MB. Gene transfection efficiency was evaluated by fluorescence microscopy and In Vivo Imaging Systems. This study demonstrated that the combination of ultrasound with PSP@MB can remarkably promote gene delivery to solid tumors as well as diminishing the toxicity towards normal tissues in vivo. The combination of PSP@MB and the use of ultrasound can efficiently enhance accumulation, extravasation and penetration into solid tumors.
Conclusion: Taken together, our study showed that this novel PSP@MB and ultrasound-mediated gene delivery system could efficiently target CSCs.
Keywords: ultrasound, PEGylated PEI-loaded microbubble, cancer stem cell, gene delivery