111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

新型氧杂蒽酮类似物的合成及其对人肺癌 A549 细胞的生长抑制活性
Authors Wu J, Dai J, Zhang Y, Wang J, Huang L, Ding H, Li T, Zhang Y, Mao J, Yu S
Received 30 May 2019
Accepted for publication 21 November 2019
Published 13 December 2019 Volume 2019:13 Pages 4239—4246
DOI https://doi.org/10.2147/DDDT.S217827
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Anastasios Lymperopoulos
Purpose: Xanthones demonstrated an array of pharmacological activities via non-covalent DNA interaction and have been widely utilized in new drug research. The introduction of the polar 1,2,3-triazole ring located at the C3-position of xanthone has not been reported thus far.
Methods: In the present study, a series of xanthone derivatives were designed, synthesized, and characterized through 1H NMR, 13C NMR, and MS. The methyl thiazolyl tetrazolium method was used to evaluate the cytotoxic activity of compounds. Furthermore, the structure–activity relationship and the potential mechanism of target compounds were investigated.
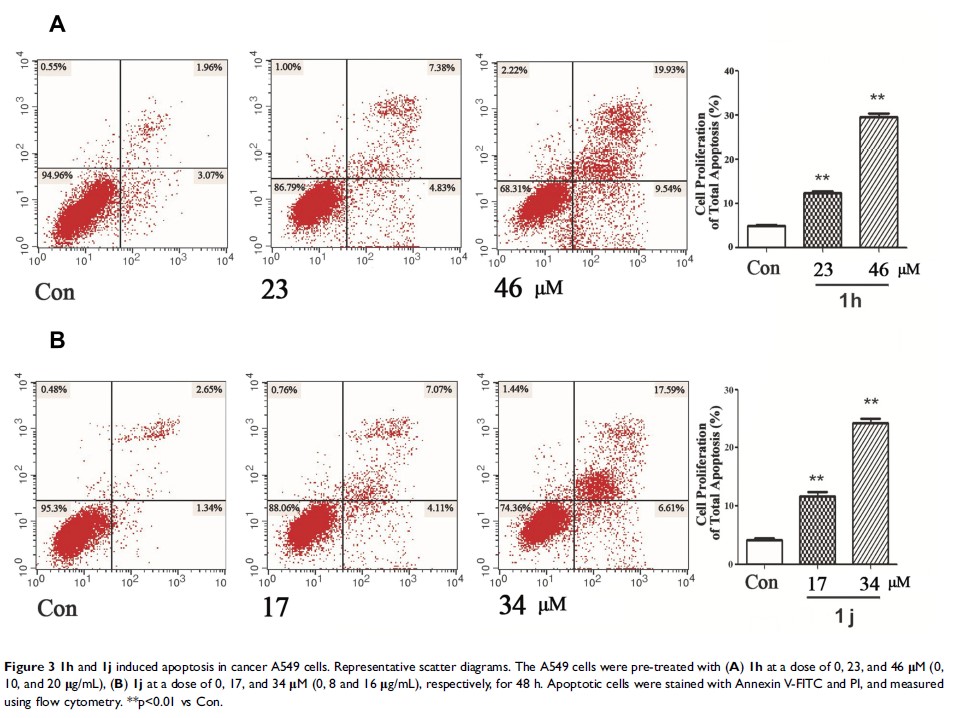
Results: The IC50 showed that the inhibitory activity of 18 target compounds was higher than that of the original xanthone intermediate 4. In particular, compound 1j was the most active agent against A549 cancer cells (IC50 = 32.4 ± 2.2 μM). Moreover, apoptosis analysis indicated different contributions of early/late apoptosis to cell death for compounds 1h and 1j. The results of Western blotting analysis showed that compound 1j significantly increased the expression of caspase 3, Bax, and c-Jun N-terminal kinase, and regulated p53 to a better healthy state in cancer cells.
Conclusion: We synthesized several derivatives of xanthone and evaluated their cytotoxicity. The evidence suggested that compound 1j possessed greater anticancer potential for further evaluations.
Keywords: synthesis, xanthone, derivatives, lung cancer cell, apoptosis