111314
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

中国开放对新型药物的获取-卫生系统视角的回顾
Authors Diao Y, Li M, Huang Z, Sun J, Chee YL, Liu Y
Received 7 August 2019
Accepted for publication 24 October 2019
Published 18 December 2019 Volume 2019:12 Pages 357—367
DOI https://doi.org/10.2147/RMHP.S226379
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 3
Editor who approved publication: Professor Marco Carotenuto
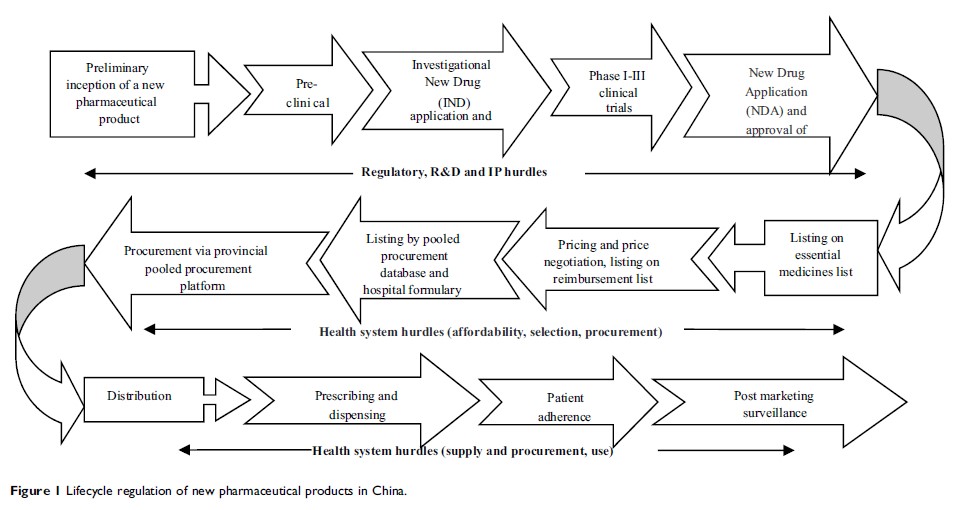
Abstract: China’s healthcare reform aims to provide affordable and equitable basic healthcare for all by 2020. Access to medicines is an essential part of the healthcare. The efforts of promoting access to medicines have been moving from meeting the needs of the basic healthcare, towards increasingly dedicated resources to offer breakthrough therapies. Looking at access to novel medicines from a health system perspective, and placing the changes China has made into that system context, this paper makes a comprehensive review of the progress of access to novel medicines in China. The review drew on two sources of information, which included desk review of published and grey literature, and key informant interview. Five hurdles were identified which create barriers of access to novel medicines, ranging from regulation and financing of medicines, intellectually property rights protection, and development of innovation capacity, to other health system components. Multiple policies have been implementing in China to remove the multiple access barriers gradually. Universal access to medicines has been moving from towards the basic common conditions to the world breakthrough technologies. We see cause for optimism, but recognize that there is a long way to go. Achieving broader and better access to modern medicines for Chinese patients will require multiple and coordinated government efforts, which would need to target the whole lifecycle regulation of novel medicines with a health system perspective, from balancing IP protection, strengthening R&D and public health, to appropriate regulatory approach and financing mechanism, and to supply chain management, as well as smart use.
Keywords: access to novel medicines, regulatory, affordability, IP, R&D, health system