110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

与人脐带血源性多能干细胞共同培养的淋巴细胞的移植可减轻缺血性卒中的炎症小体活性
Authors Zhao Y, Zhu T, Li H, Zhao J, Li X
Received 17 July 2019
Accepted for publication 11 December 2019
Published 19 December 2019 Volume 2019:14 Pages 2261—2271
DOI https://doi.org/10.2147/CIA.S223595
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Background: Manipulating the immune inflammatory response after cerebral ischemia has been a novel therapeutic strategy for ischemic stroke. This study attempted to investigate the effects of the transplantation of lymphocytes co-cultured with human cord blood-derived multipotent stem cells (HCB-SCs) on the inflammatory response in transient middle cerebral occlusion (tMCAO) rats.
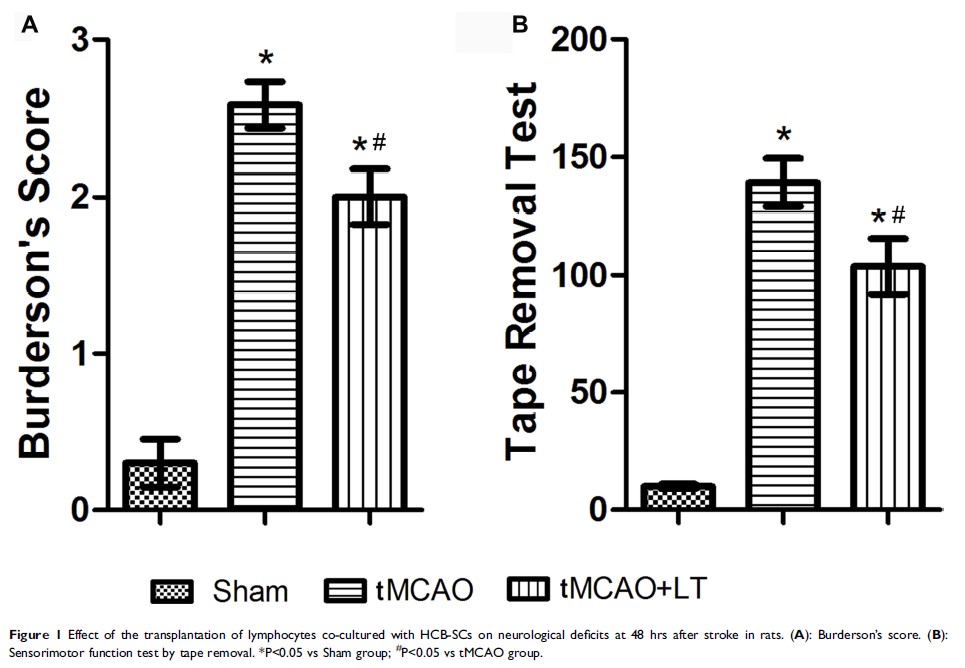
Methods: The tMCAO rats were subjected to the transplantation of lymphocytes co-cultured with HCB-SCs through tail vein injection. Infarct size and neurological deficits were measured at 48 hrs after stroke. Neurological deficits were assessed using Bederson’s scoring system and tape removal test. Blood T cell flow cytometry was performed to measure the differentiation of regulatory T cells (Tregs). Western blot was used to detect the protein levels of inflammation-related molecules, apoptosis-related molecule, and signaling molecules in ischemic brain. TUNEL staining was performed to analyze cell apoptosis in ischemic cerebral cortex.
Results: The transplantation of lymphocytes co-cultured with HCB-SCs significantly improved the neurological defects, reduced ischemic brain damage, and increased the proportion of peripheral CD4+CD25+Foxp3+ Tregs. Meanwhile, the transplantation of co-cultured cells decreased the expression of NLRP3 inflammasome and associated factors, such as caspase-1 and IL-1β, and inhibited the activation of NF-κB, ERK and caspase-3 in ischemic brain. The co-cultured cells significantly decreased the number of tMCAO-induced cell apoptosis.
Conclusion: Lymphocytes co-cultured with HCB-SCs exhibit a neuroprotective effect after ischemic stroke by promoting Tregs differentiation and suppressing NLRP3 inflammasome activation and neuron apoptosis, and might be a promising therapeutic strategy for ischemic stroke.
Keywords: ischemic stroke, inflammation, cord blood-derived multipotent stem cells, regulatory T-cells, inflammasomes