110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

由化疗导致的 Mcl-1 表达增加可介导 miR-375 对骨肉瘤细胞顺铂耐药性的影响
Authors Liu A, Yu H, Yang Y, Xue F, Chen X, Zhang Y, Zhou Z, Zhang B, Li L, Sun C, Huang P, Huang J
Received 15 September 2019
Accepted for publication 13 December 2019
Published 31 December 2019 Volume 2019:12 Pages 11667—11677
DOI https://doi.org/10.2147/OTT.S231125
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Background: Osteosarcoma (OS) is one of the most difficult cancers to treat due to its resistance to chemotherapy. The essential role played by Mcl-1 in promoting chemoresistance has been observed in a variety of cancers, including OS, while the underlying mechanism remains unclear.
Methods: We investigated the expression of Mcl-1 in 42 paired OS specimens obtained before and after adjuvant chemotherapy, and its correlation with clinicopathological characteristics. Loss and gain of function studies were performed to analyze the effects of Mcl-1 modulations on the chemosensitivity, and the mechanism involved in the deregulation of Mcl-1 in OS cells.
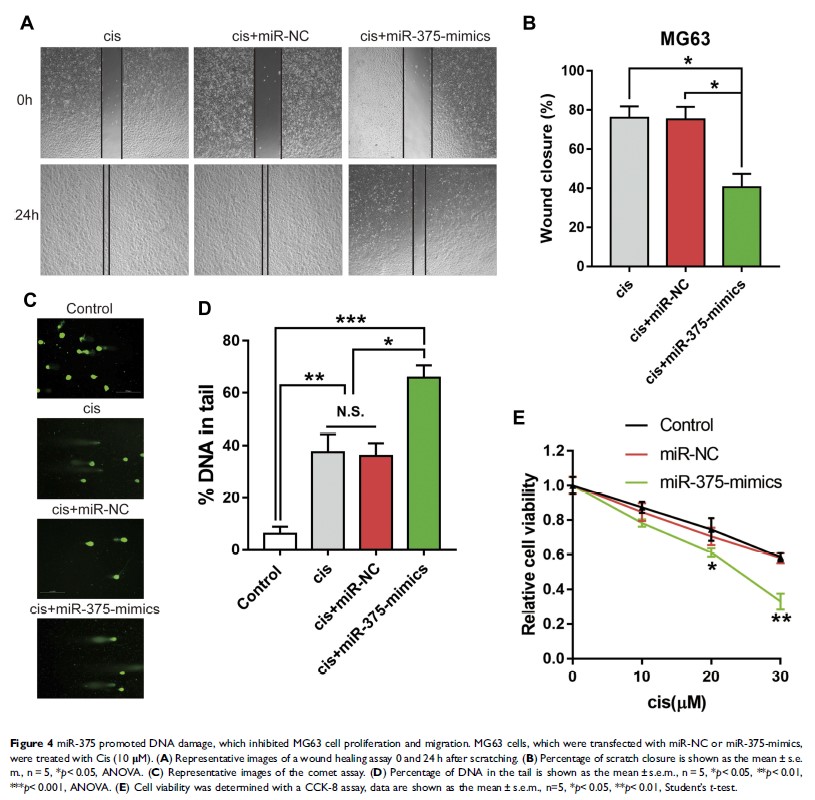
Results: In OS specimens, the expression of Mcl-1 was significantly upregulated after chemotherapy, and high Mcl-1 expression was associated with poorer overall survival and an increased recurrence rate. Furthermore, we demonstrated that chemotherapy-driven increased Mcl-1 decreased chemosensitivity by promoting tumour proliferation and inhibiting DNA damage. Moreover, Mcl-1 was found to be a direct target of miR-375 in OS cells. The knockdown of Mcl-1 phenocopied miR-375 downregulation, and the overexpression of miR-375 rescued the effects of cisplatin-induced DNA damage mediated by Mcl-1.
Conclusion: Our data indicated that chemotherapy-driven increase in the expression of Mcl-1 plays a critical role in chemoresistance, and the intervention of the miR-375/Mcl-1 axis may offer a novel strategy to enhance chemosensitivity in OS treatment.
Keywords: osteosarcoma, Mcl-1, chemotherapy resistance, miR-375, cisplatin